
How to remove Nitro Group from nitrobenzene ring?
Answer
549.3k+ views
Hint: Reactions used in these questions are Reduction Reaction, diazotize and deaminate reaction. In reduction reactions the compound is reduced by gaining electrons in presence of acid. In diazotization. The aromatic primary amine is converted to its corresponding diazonium salt and is commonly referred as diazotization (use of nitrous acid in presence of another acid) In diminution the amine group is removed.
Complete step by step answer:
STEP 1 — Reduction
Nitrobenzene is reduced to phenyl ammonium ion using a mixture of tin and cone. HCl. The mixture is heated under reflux in a boiling water both for about half an hour
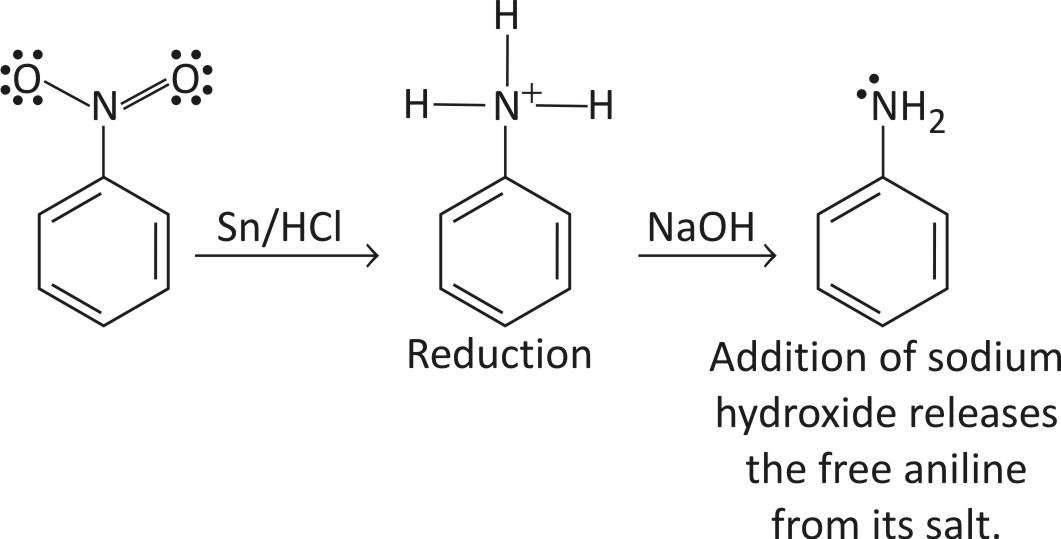
STEP 2 — Diazotization
Amino benzene is converted to a diazonium ion with $\text{HN}{{\text{O}}_{3}}\text{ or HCl}$. In this reaction primary aromatic amine is converted into a diazonium salt of the amine.
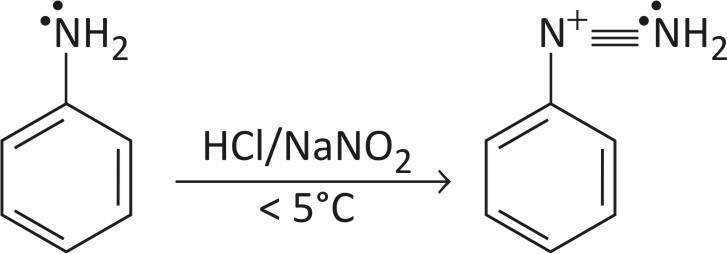
It is done at a temperature of less than $5{}^\circ \text{C}$ to form a stable compound by resonance.
STEP 3 — Deamination
In deamination, nitrogen is removed by heating diazonium salt reacts with cold aq. Hypo phosphorous acid to evolve nitrogen and form benzene.
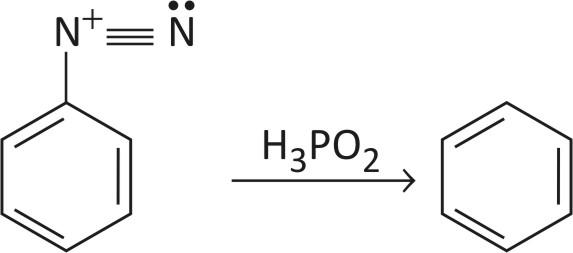
This is how nitro groups are removed from benzene.
Note: It is one of the nucleophilic aromatic substitution reactions.
Reduction of nitro aromatics is conducted on an industrial scale. Many methods exist.
Catalytic hydrogenation using nickel/palladium
Iron in acid media, etc.
Diazonium compounds are used in dyes and pigment industries, they are also used as standard reagents.
Deamination reaction takes place in the liver and kidney.
Complete step by step answer:
STEP 1 — Reduction
Nitrobenzene is reduced to phenyl ammonium ion using a mixture of tin and cone. HCl. The mixture is heated under reflux in a boiling water both for about half an hour

STEP 2 — Diazotization
Amino benzene is converted to a diazonium ion with $\text{HN}{{\text{O}}_{3}}\text{ or HCl}$. In this reaction primary aromatic amine is converted into a diazonium salt of the amine.

It is done at a temperature of less than $5{}^\circ \text{C}$ to form a stable compound by resonance.
STEP 3 — Deamination
In deamination, nitrogen is removed by heating diazonium salt reacts with cold aq. Hypo phosphorous acid to evolve nitrogen and form benzene.

This is how nitro groups are removed from benzene.
Note: It is one of the nucleophilic aromatic substitution reactions.
Reduction of nitro aromatics is conducted on an industrial scale. Many methods exist.
Catalytic hydrogenation using nickel/palladium
Iron in acid media, etc.
Diazonium compounds are used in dyes and pigment industries, they are also used as standard reagents.
Deamination reaction takes place in the liver and kidney.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




