
Resonance hybrid of nitrate ion is ……….
A.
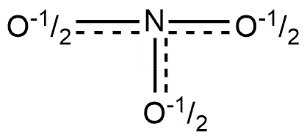
B.

C.

D.
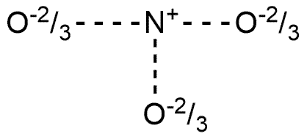



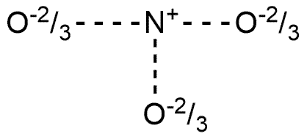
Answer
561k+ views
Hint: In chemistry resonance can be defined as a way of describing bonding in certain molecules or ions by the combination of different contributing structures and the structures formed during the process of resonance are known as resonating or canonical structures.
Complete step by step answer:
- During the process of resonance resonating structure turned into a resonance hybrid also known as hybrid structure. It contains a particular value which generally describes delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis structure.
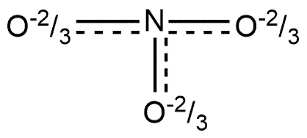
Lewis structure of nitrate ion can be shown by:
- According to resonance theory each bond in the nitrate ion is one and one third of a bond present between nitrogen and oxygen which is consistent with the observation that the three bonds in the nitrate ion have the same bond length and the same bond energy. It can be explained as we know that oxygen carries -1 charge in this case and nitrogen carries neutral charge so average charge of $O-N-O$ is given by
$\begin{align}
& 1\to -1 \\
& 2\to 0 \\
& 3\to -1 \\
\end{align}$
Here 1 and 3 refers with $O$ and 2 refers with $N$. Weighted average will be given by:
$(-1\times \dfrac{1}{3})+(0\times \dfrac{1}{3})+(-1\times \dfrac{1}{3})=\dfrac{-2}{3}$
In all three cases the weighted average will remain the same i.e. $\dfrac{-2}{3}$.
The correct answer is option “B” .
Note: Hybrid is a compound, molecule, ion or radical exhibiting the property of resonance and having a structure which can be represented in the written form as the average of two or more structural formulas separated each from the next by a double-headed arrow.
Complete step by step answer:
- During the process of resonance resonating structure turned into a resonance hybrid also known as hybrid structure. It contains a particular value which generally describes delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis structure.
Lewis structure of nitrate ion can be shown by:

- According to resonance theory each bond in the nitrate ion is one and one third of a bond present between nitrogen and oxygen which is consistent with the observation that the three bonds in the nitrate ion have the same bond length and the same bond energy. It can be explained as we know that oxygen carries -1 charge in this case and nitrogen carries neutral charge so average charge of $O-N-O$ is given by
$\begin{align}
& 1\to -1 \\
& 2\to 0 \\
& 3\to -1 \\
\end{align}$
Here 1 and 3 refers with $O$ and 2 refers with $N$. Weighted average will be given by:
$(-1\times \dfrac{1}{3})+(0\times \dfrac{1}{3})+(-1\times \dfrac{1}{3})=\dfrac{-2}{3}$
In all three cases the weighted average will remain the same i.e. $\dfrac{-2}{3}$.
The correct answer is option “B” .
Note: Hybrid is a compound, molecule, ion or radical exhibiting the property of resonance and having a structure which can be represented in the written form as the average of two or more structural formulas separated each from the next by a double-headed arrow.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




