
What is reverse osmosis? What is its practical utility?
Answer
582.9k+ views
Hint: Reverse osmosis is an exact reverse process of osmosis. In osmosis, we do not give any external pressure to the solutions, while in reverse osmosis; we apply a certain amount of external pressure on the concentrated solution.
Complete step by step solution:
Reverse osmosis is a process which is exactly reverse to the osmosis. We know that in osmosis, the solvent flows from less concentrated side to the side which has a higher concentration of solutes. In reverse osmosis, we reverse the flow of solvent and this is made possible by applying external pressure on the concentrated solution.
- We need to give such a pressure which is larger than the osmotic pressure to the side which has a higher concentration of solutes.
- So, here, the pure solvent will flow through the semipermeable membrane. We can use porous membranes such as a film of cellulose acetate placed over certain suitable support. Cellulose acetate can flow water through it but it is impermeable to the ions and other impurities in the water. The diagram of reverse osmosis is given below.
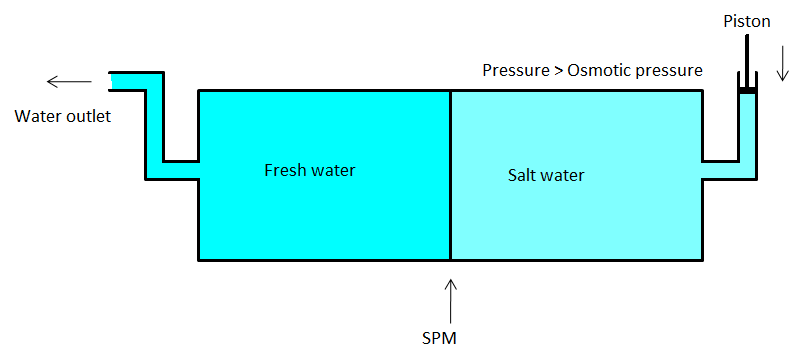
The practical utility of Reverse osmosis:
- Reverse osmosis is used in the desalination of seawater. Desalination of water is the removal of salts from the seawater which has plenty of salts dissolved.
- We can use various semi-permeable membranes for this purpose. With this method, may countries can now produce the required drinking water from seawater.
Note: Do not get confused between the flow of solvent in osmosis and reverse osmosis. In osmosis, the solvent flows from a less concentrated solution to a more concentrated solution. In reverse osmosis, the flow of solvent is from a more concentrated solution to a less concentrated solution.
Complete step by step solution:
Reverse osmosis is a process which is exactly reverse to the osmosis. We know that in osmosis, the solvent flows from less concentrated side to the side which has a higher concentration of solutes. In reverse osmosis, we reverse the flow of solvent and this is made possible by applying external pressure on the concentrated solution.
- We need to give such a pressure which is larger than the osmotic pressure to the side which has a higher concentration of solutes.
- So, here, the pure solvent will flow through the semipermeable membrane. We can use porous membranes such as a film of cellulose acetate placed over certain suitable support. Cellulose acetate can flow water through it but it is impermeable to the ions and other impurities in the water. The diagram of reverse osmosis is given below.

The practical utility of Reverse osmosis:
- Reverse osmosis is used in the desalination of seawater. Desalination of water is the removal of salts from the seawater which has plenty of salts dissolved.
- We can use various semi-permeable membranes for this purpose. With this method, may countries can now produce the required drinking water from seawater.
Note: Do not get confused between the flow of solvent in osmosis and reverse osmosis. In osmosis, the solvent flows from a less concentrated solution to a more concentrated solution. In reverse osmosis, the flow of solvent is from a more concentrated solution to a less concentrated solution.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




