
Ribose sugar differs from deoxyribose in having
A. two extra oxygen molecules
B.one extra oxygen molecules
C.no oxygen
D. hydroxyl group
Answer
555k+ views
Hint: To answer this question we should know the structure of ribose and deoxyribose sugar. If we know the structure, we can easily tell the difference between these two sugars. The chemical formula of ribose sugar is ${{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}_{\text{5}}}$ and the chemical formula of deoxyribose sugar is ${{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}_4}$.
Complete step-by-step answer:Ribose and deoxyribose both are carbohydrate. The large organic molecule made up of carbon, hydrogen, and oxygen only are known as carbohydrates or sugars. Carbohydrates have hydrogen and oxygen in $2:1$ ratio. Ribose and deoxyribose sugar both are nucleic acids.
Ribose sugar and deoxyribose both are the same. Both sugars contain five membered rings. Both differ only in the presence of one hydroxyl group.
The structure of the ribose and deoxyribose sugars are as follows:
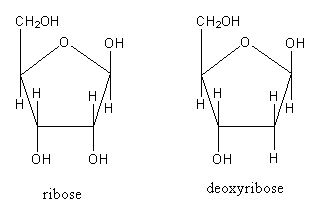
At second carbon ribose sugar has one hydrogen and one hydroxyl group whereas at second carbon the deoxyribose sugar has two hydrogens means the deoxyribose has one less hydroxy group. As the deoxyribose sugar lacks one hydroxyl group so it is known as deoxy.
So, ribose sugar differs from deoxyribose in having hydroxyl group.
Therefore, option (D) hydroxyl group, is correct.
Note: Instead of having one hydroxyl group, deoxyribose sugar has only one hydrogen group. Ribose sugar is found in ribonucleic acid RNA whereas deoxyribose sugar is found in deoxyribonucleic acid DNA. The absence of the hydroxyl group is significant for the extra stability of the DNA. The DNA contains the adenine, thiamine, cytosine, and guanine base whereas the RNA contains uracil in place of adenine.
Complete step-by-step answer:Ribose and deoxyribose both are carbohydrate. The large organic molecule made up of carbon, hydrogen, and oxygen only are known as carbohydrates or sugars. Carbohydrates have hydrogen and oxygen in $2:1$ ratio. Ribose and deoxyribose sugar both are nucleic acids.
Ribose sugar and deoxyribose both are the same. Both sugars contain five membered rings. Both differ only in the presence of one hydroxyl group.
The structure of the ribose and deoxyribose sugars are as follows:

At second carbon ribose sugar has one hydrogen and one hydroxyl group whereas at second carbon the deoxyribose sugar has two hydrogens means the deoxyribose has one less hydroxy group. As the deoxyribose sugar lacks one hydroxyl group so it is known as deoxy.
So, ribose sugar differs from deoxyribose in having hydroxyl group.
Therefore, option (D) hydroxyl group, is correct.
Note: Instead of having one hydroxyl group, deoxyribose sugar has only one hydrogen group. Ribose sugar is found in ribonucleic acid RNA whereas deoxyribose sugar is found in deoxyribonucleic acid DNA. The absence of the hydroxyl group is significant for the extra stability of the DNA. The DNA contains the adenine, thiamine, cytosine, and guanine base whereas the RNA contains uracil in place of adenine.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




