
What is the role of pyridine in the acylation of amines?
Answer
512.7k+ views
Hint :in the given question we should know about the chemical reaction of the acylation of amines. When the acyl group is added to a molecule, that type of chemical reaction is called an acylation reaction. Amines are the organic compounds which contain a nitrogen atom with a lone pair of electrons. Acylation of amines is the reaction of amines with the acetyl chloride.
Complete Step By Step Answer:
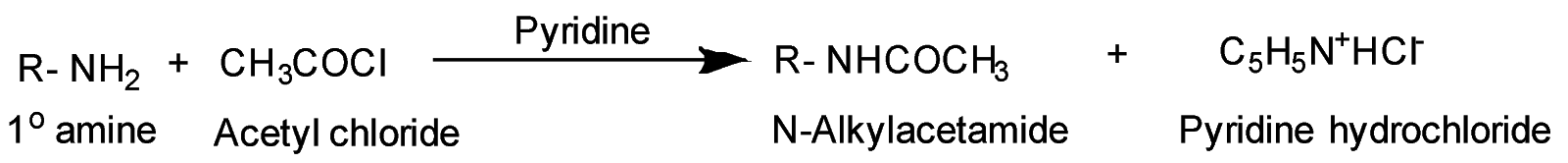
Acylation of amines is the reaction between the amines ( $ - N{H_2} $ group) and the acetyl group ( $ C{H_3}CO - $ ) that comes from either acetyl halide or acetic anhydride. This reaction takes place in the presence of pyridine ( $ {C_5}{H_5}N $ ) which acts as a strong base during the reaction. During substitution reaction of acylation of ( $ - N{H_2} $ ) group with acetyl group followed by hydrolysis of substituted amide with substituted amine, the activating effect of $ - N{H_2} $ group can be controlled. Pyridine is used to remove the side product formed in the acylation reaction i.e. $ HCl $ from the reaction mixture. It acts as an acceptor for the acid byproduct formed in the reaction.
Pyridine also acts as a nucleophile for the carbonyl group due to its lone pair of electrons on nitrogen atoms which can be delocalized in its ring. It acts as a catalyst and is often used in the acylation reactions.
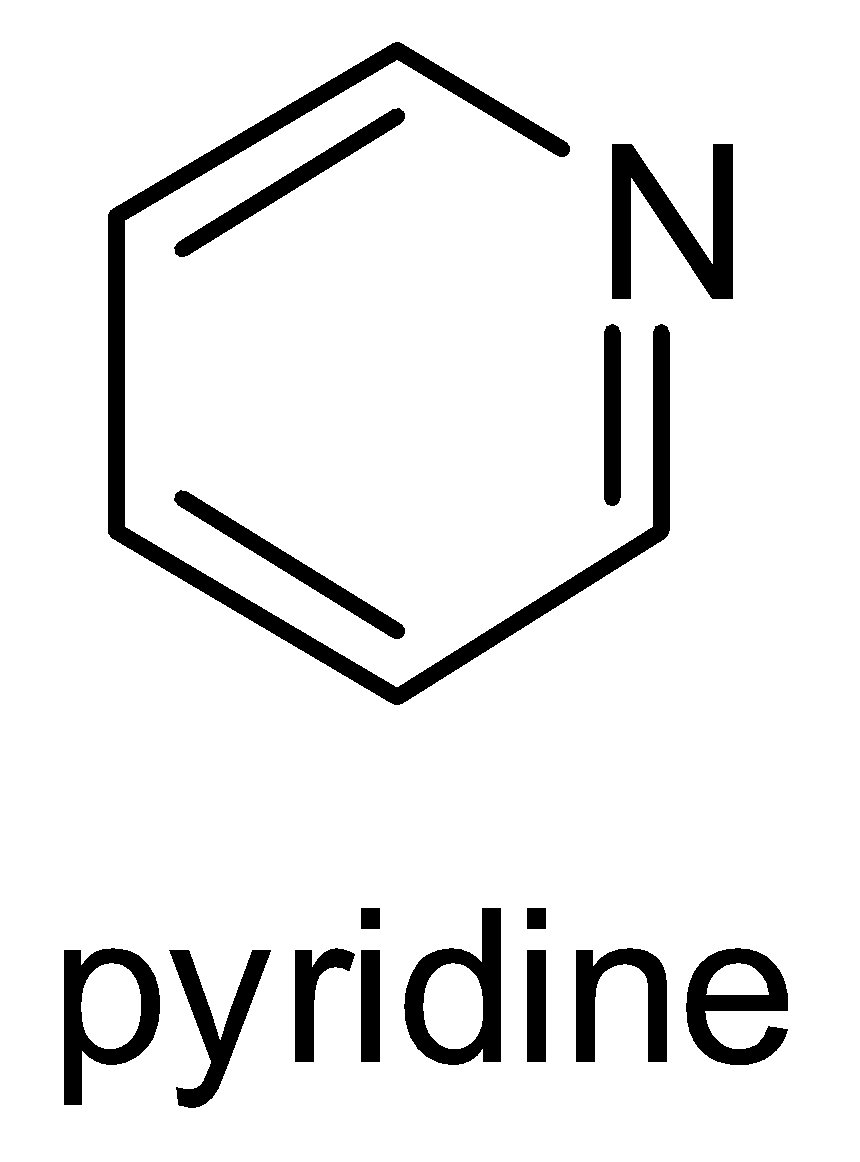
Structure of Pyridine-
Example- Chemical Reaction between primary amine and acetyl chloride
Note :
It needs to be remembered that pyridine is a basic heterocyclic organic compound that acts as a base as well as nucleophile. It has a lone pair of electrons on nitrogen atoms which get delocalized in the ring. Structure of pyridine and its molecular formula ( $ {C_5}{H_5}N $ ) should be remembered. Pyridine is a water- miscible liquid with a distinctive and unpleasant fish like smell. It is a highly flammable and weakly alkaline liquid.
Complete Step By Step Answer:
Acylation of amines is the reaction between the amines ( $ - N{H_2} $ group) and the acetyl group ( $ C{H_3}CO - $ ) that comes from either acetyl halide or acetic anhydride. This reaction takes place in the presence of pyridine ( $ {C_5}{H_5}N $ ) which acts as a strong base during the reaction. During substitution reaction of acylation of ( $ - N{H_2} $ ) group with acetyl group followed by hydrolysis of substituted amide with substituted amine, the activating effect of $ - N{H_2} $ group can be controlled. Pyridine is used to remove the side product formed in the acylation reaction i.e. $ HCl $ from the reaction mixture. It acts as an acceptor for the acid byproduct formed in the reaction.
Pyridine also acts as a nucleophile for the carbonyl group due to its lone pair of electrons on nitrogen atoms which can be delocalized in its ring. It acts as a catalyst and is often used in the acylation reactions.
Structure of Pyridine-

Example- Chemical Reaction between primary amine and acetyl chloride

Note :
It needs to be remembered that pyridine is a basic heterocyclic organic compound that acts as a base as well as nucleophile. It has a lone pair of electrons on nitrogen atoms which get delocalized in the ring. Structure of pyridine and its molecular formula ( $ {C_5}{H_5}N $ ) should be remembered. Pyridine is a water- miscible liquid with a distinctive and unpleasant fish like smell. It is a highly flammable and weakly alkaline liquid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




