
Salicylaldehyde can be prepared from phenol by:
A. Schotten-Baumann reaction
B. Kolbe’s reaction
C. Relmer-Tiemann reaction
D. Perkin reaction
E. Balz Schiemann reaction
Answer
582k+ views
Hint: Salicylaldehyde is an organic compound with chemical formula ${C_7}{H_6}{O_2}$. It is one of the three isomers of hydroxybenzaldehyde. Phenol and chloroform are heated together in the presence of sodium hydroxide and potassium hydroxide to form Salicylaldehyde.
Step by step answer: Salicylaldehyde is the organic compound with formula ${C_7}{H_6}{O_2}\left( {{C_6}{H_4}CHO - 2 - OH} \right)$. It is a colorless oily liquid which has a bitter almond odor at high concentrations. It is also known as 2-Hydroxybenzaldehyde. It is used in the production of coumarin, saligenin, in analytical chemistry etc.
The molecular formula of Phenol is ${C_6}{H_5}OH$. Phenol is an aromatic organic compound. It is a white crystalline volatile solid.
The molecular form of chloroform is $CHC{l_3}$. It is an artificial product and a colorless liquid with a non-irritating smell and slightly sweet taste.
Salicylaldehyde is prepared by heating phenol and chloroform together in the presence of potassium hydroxide and sodium hydroxide. This reaction is called the Relmer-Tiemann reaction.
${C_6}{H_5}OH + CHC{l_3}\xrightarrow{{3KOH}}{C_7}{H_6}{O_2}$
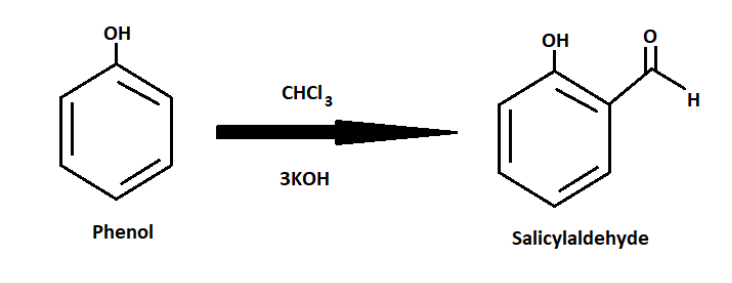
The correct option is Option C, Reimer-Tiemann reaction.
Note: Phenol is a mild acid and can cause chemical burns, so it must be handled carefully. Salicylaldehyde is 2-Hydroxybenzaldehyde. 4- Hydroxybenzaldehyde is an organic compound with the same chemical formula as Salicylaldehyde but different structure, where hydroxyl group is present at the 4th carbon. Benzoic acid also has the same molecular formula as Salicylaldehyde, but with a carboxyl group. So be careful with the compounds when their IUPAC names are not given.
Step by step answer: Salicylaldehyde is the organic compound with formula ${C_7}{H_6}{O_2}\left( {{C_6}{H_4}CHO - 2 - OH} \right)$. It is a colorless oily liquid which has a bitter almond odor at high concentrations. It is also known as 2-Hydroxybenzaldehyde. It is used in the production of coumarin, saligenin, in analytical chemistry etc.
The molecular formula of Phenol is ${C_6}{H_5}OH$. Phenol is an aromatic organic compound. It is a white crystalline volatile solid.
The molecular form of chloroform is $CHC{l_3}$. It is an artificial product and a colorless liquid with a non-irritating smell and slightly sweet taste.
Salicylaldehyde is prepared by heating phenol and chloroform together in the presence of potassium hydroxide and sodium hydroxide. This reaction is called the Relmer-Tiemann reaction.
${C_6}{H_5}OH + CHC{l_3}\xrightarrow{{3KOH}}{C_7}{H_6}{O_2}$

The correct option is Option C, Reimer-Tiemann reaction.
Note: Phenol is a mild acid and can cause chemical burns, so it must be handled carefully. Salicylaldehyde is 2-Hydroxybenzaldehyde. 4- Hydroxybenzaldehyde is an organic compound with the same chemical formula as Salicylaldehyde but different structure, where hydroxyl group is present at the 4th carbon. Benzoic acid also has the same molecular formula as Salicylaldehyde, but with a carboxyl group. So be careful with the compounds when their IUPAC names are not given.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




