
Same osazone derivative is obtained in case of D-glucose, D-Mannose and D Fructose due to:
A.the same configuration at C-5
B. B the same constitution
C. the same constitution at C-1 and C-2
D. The same constitution and configuration at C-3, C-4, C-5 and C-6 but different constitution and configuration at C-1 and C-2 which becomes identical by osazone formation
Answer
566.4k+ views
Hint: D, L System of Configurational Designation : The letters 'D' & 'l' before the name of any compound indicate the substituents orientation at a centre of chirality to that in D- and L-Glyceraldehydes. Glyceraldehyde contains one carbon atom that is asymmetric and exists in two enantiomeric forms.
Complete step by step answer:
The D- and L- nomenclature to glyceraldehyde was arbitrarily given by Fischer who introduced this system D-refers to an arrangement about a centre of chirality that is identical to the three dimensional arrangement in D-(+) glyceraldehyde where we can observe the -OH group present in the chiral centre is on right in its Fischer projection. Similarly talking about L, to an arrangement about a centre of chirality that is identical to the 3D-assigned in L-glyceraldehyde. All molecules which could be chemically related to D-glyceraldehyde are arranged the D-configuration and those related to L-glyceraldehyde are assigned L-configuration. For assigning the configuration of monosaccharides, it is the lowest asymmetric carbon atom (in the Fischer projection formula of the compound) is compared,
The two cyclic hemiacetal forms of glucose have their difference only in the configuration of the C-1 hydroxyl group , called anomeric carbon (the aldehydic carbon before cycle formation) and the corresponding a and B-forms are called anomers. It should be noted that a and B-forms of glucose are not mirror images of each other, hence are not enantiomers. As it is analogous with pyran the six membered cyclic structure of glucose is called pyranose structure.
Pyran is a six membered ring with one oxygen and five carbon atoms in the ring.
Action of Phenylhydrazine (Osazone Formation):
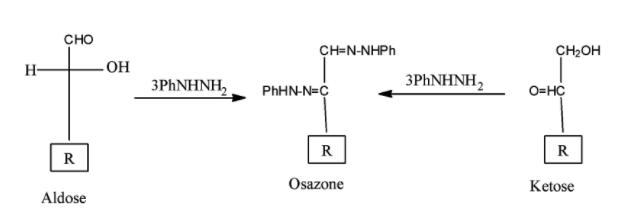
Hence, our correct answer for the question is option D.
Note: When aldose or ketose reacts with three moles of phenyl hydrazine and Osazone is formed. Aldose and its C-2 epimer give the same osazone. Similarly Aldose & Ketose having identical configuration from C-3 will give the same osazone.
Complete step by step answer:
The D- and L- nomenclature to glyceraldehyde was arbitrarily given by Fischer who introduced this system D-refers to an arrangement about a centre of chirality that is identical to the three dimensional arrangement in D-(+) glyceraldehyde where we can observe the -OH group present in the chiral centre is on right in its Fischer projection. Similarly talking about L, to an arrangement about a centre of chirality that is identical to the 3D-assigned in L-glyceraldehyde. All molecules which could be chemically related to D-glyceraldehyde are arranged the D-configuration and those related to L-glyceraldehyde are assigned L-configuration. For assigning the configuration of monosaccharides, it is the lowest asymmetric carbon atom (in the Fischer projection formula of the compound) is compared,
The two cyclic hemiacetal forms of glucose have their difference only in the configuration of the C-1 hydroxyl group , called anomeric carbon (the aldehydic carbon before cycle formation) and the corresponding a and B-forms are called anomers. It should be noted that a and B-forms of glucose are not mirror images of each other, hence are not enantiomers. As it is analogous with pyran the six membered cyclic structure of glucose is called pyranose structure.
Pyran is a six membered ring with one oxygen and five carbon atoms in the ring.
Action of Phenylhydrazine (Osazone Formation):

Hence, our correct answer for the question is option D.
Note: When aldose or ketose reacts with three moles of phenyl hydrazine and Osazone is formed. Aldose and its C-2 epimer give the same osazone. Similarly Aldose & Ketose having identical configuration from C-3 will give the same osazone.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




