
What is the Saytzeff rule, illustrated with an example.
Answer
507.9k+ views
Hint :Saytzeff rule is applicable in the case of haloalkanes where an elimination reaction needs to be carried out in such a way that it leads to an unsymmetrical alkene formation. The choice of hydrogen depends upon the stability of the product.
Complete Step By Step Answer:
Hydrocarbons undergo a variety of organic reactions. One such reaction shown by haloalkanes is the elimination reaction. As the name suggests, the reaction involves the removal of a halogen atom attached to a carbon center and the removal of a hydrogen atom attached to a carbon atom placed adjacent to the halogen bonded carbon. This reaction is also referred to as dehydrohalogenation reaction and results in the formation of a double bond between the carbon atoms involved.
If the carbon containing the halogen atom is placed in a symmetrical environment i.e. the carbon atoms placed adjacent to it are equally substituted then the hydrogen removal can take place from either side.
When a hydrogen has to be removed from a haloalkane molecule with unequal substituted carbon atoms, then the nature of hydrogens needs to be compared in order to remove the suitable hydrogen.
This comparison is based on the Saytzeff’s rule that suggests that a hydrogen needs to be removed from the more substituted carbon atom in order to achieve higher stability of the alkene being formed as a result of elimination. A more substituted carbon atom is the one which has lesser number of hydrogens attached to it and more number of alkyl groups.
Thus the preference rule for hydrogen removal attached to a carbon atom is:
$ tertiary > \secondary > primary $
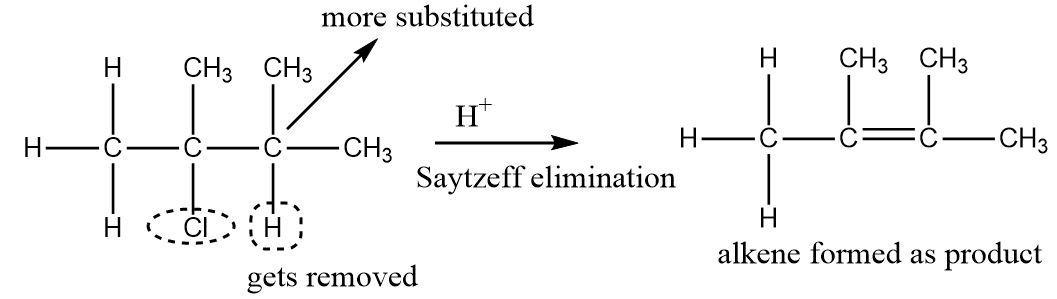
An example of Saytzeff elimination can be seen as follows:
Note :
The reason for Saytzeff’s elimination rule being successful in giving more stable alkene products is hyperconjugation. A more substituted alkene contains a double bond surrounded by more numbers of $ \alpha - hydrogens $ , which enhance the stability of the alkene.
Complete Step By Step Answer:
Hydrocarbons undergo a variety of organic reactions. One such reaction shown by haloalkanes is the elimination reaction. As the name suggests, the reaction involves the removal of a halogen atom attached to a carbon center and the removal of a hydrogen atom attached to a carbon atom placed adjacent to the halogen bonded carbon. This reaction is also referred to as dehydrohalogenation reaction and results in the formation of a double bond between the carbon atoms involved.
If the carbon containing the halogen atom is placed in a symmetrical environment i.e. the carbon atoms placed adjacent to it are equally substituted then the hydrogen removal can take place from either side.
When a hydrogen has to be removed from a haloalkane molecule with unequal substituted carbon atoms, then the nature of hydrogens needs to be compared in order to remove the suitable hydrogen.
This comparison is based on the Saytzeff’s rule that suggests that a hydrogen needs to be removed from the more substituted carbon atom in order to achieve higher stability of the alkene being formed as a result of elimination. A more substituted carbon atom is the one which has lesser number of hydrogens attached to it and more number of alkyl groups.
Thus the preference rule for hydrogen removal attached to a carbon atom is:
$ tertiary > \secondary > primary $
An example of Saytzeff elimination can be seen as follows:

Note :
The reason for Saytzeff’s elimination rule being successful in giving more stable alkene products is hyperconjugation. A more substituted alkene contains a double bond surrounded by more numbers of $ \alpha - hydrogens $ , which enhance the stability of the alkene.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




