
Why ${\text{S}}{{\text{c}}^{{\text{3 + }}}}$salts are colourless whereas ${\text{C}}{{\text{r}}^{{\text{3 + }}}}$salts are coloured?
Answer
550.8k+ views
Hint: Crystal field theory describes the removal of the degeneracy of d-orbitals of metal in presence of ligands. By the removal of degeneracy, the d-orbital of metal splits. The splitting explains many properties of transition metals like colours. The colour is produced by d-d transition. For d-d transition, unpaired electrons are required.
Complete step-by-step answer:
We will write the electronic configuration to determine the presence of electrons as follows:
Scandium is a transition element with atomic number $21$. Its valence electronic configuration is, ${\text{3}}{{\text{d}}^1}{\text{4}}{{\text{s}}^{\text{2}}}$ .
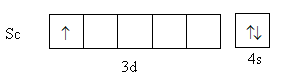
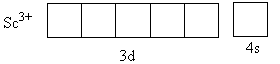
The charge on the metal is $ + 3$. The valence electronic configuration of ${\text{S}}{{\text{c}}^{{\text{3 + }}}}$ is ${\text{3}}{{\text{d}}^{\text{0}}}{\text{4}}{{\text{s}}^{\text{0}}}$,
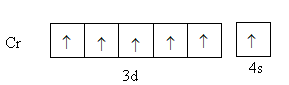
Chromium is a transition element with atomic number $24$. Its valence electronic configuration is, ${\text{3}}{{\text{d}}^5}{\text{4}}{{\text{s}}^1}$ .
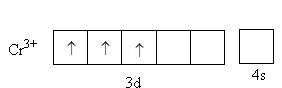
The charge on the metal is $ + 3$. The valence electronic configuration of ${\text{C}}{{\text{r}}^{{\text{3 + }}}}$ is \[{\text{3}}{{\text{d}}^3}{\text{4}}{{\text{s}}^{\text{0}}}\],
When ligands approach the metal, a complex forms. Metal’s d-orbitals split into two sets of two and three orbitals. In octahedral geometry of the complex, ligand approach from the axis and the orbitals ${{\text{d}}_{{{\text{x}}^{\text{2}}} - {{\text{y}}^{\text{2}}}}}$ and ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$ are present on the axis whereas${{\text{d}}_{{\text{xy}}}}$,${{\text{d}}_{{\text{zy}}}}$ and ${{\text{d}}_{{\text{xz}}}}$ orbitals lie in between the axes. So, the energy of two orbitals ${{\text{d}}_{{{\text{x}}^{\text{2}}} - {{\text{y}}^{\text{2}}}}}$ and ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$ increases due to the ligands and the energy of three orbitals decreases. As a result, five degenerate-orbitals lose that degeneracy and split into two groups.
The CFT diagram of the ${\text{S}}{{\text{c}}^{{\text{3 + }}}}$and ${\text{C}}{{\text{r}}^{{\text{3 + }}}}$is as follows:
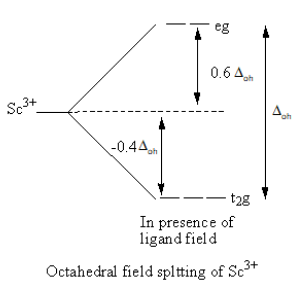
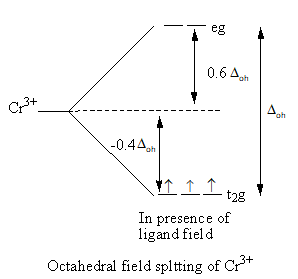
From the CFT diagram, we can say that the ${\text{S}}{{\text{c}}^{{\text{3 + }}}}$ has no d-electron so, no d-d transition is possible so, the ${\text{S}}{{\text{c}}^{{\text{3 + }}}}$is colourless whereas the ${\text{C}}{{\text{r}}^{{\text{3 + }}}}$has three unpaired electrons so, the d-d transition is possible so, the ${\text{C}}{{\text{r}}^{{\text{3 + }}}}$is coloured.
Note: Colour of $3{\text{d}} - $transition metals depend upon the d-d-transition and number of unpaired electrons in d-orbitals. Transmitted colour appears as colour of substance due to the presence of electrons that are responsible for transition. The presence of unpaired electrons also tells the magnetic nature of the complex. The given titanium complex is paramagnetic due to the presence of one unpaired electron. If all the electrons are paired then the complex will be diamagnetic.
Complete step-by-step answer:
We will write the electronic configuration to determine the presence of electrons as follows:
Scandium is a transition element with atomic number $21$. Its valence electronic configuration is, ${\text{3}}{{\text{d}}^1}{\text{4}}{{\text{s}}^{\text{2}}}$ .

The charge on the metal is $ + 3$. The valence electronic configuration of ${\text{S}}{{\text{c}}^{{\text{3 + }}}}$ is ${\text{3}}{{\text{d}}^{\text{0}}}{\text{4}}{{\text{s}}^{\text{0}}}$,

Chromium is a transition element with atomic number $24$. Its valence electronic configuration is, ${\text{3}}{{\text{d}}^5}{\text{4}}{{\text{s}}^1}$ .

The charge on the metal is $ + 3$. The valence electronic configuration of ${\text{C}}{{\text{r}}^{{\text{3 + }}}}$ is \[{\text{3}}{{\text{d}}^3}{\text{4}}{{\text{s}}^{\text{0}}}\],

When ligands approach the metal, a complex forms. Metal’s d-orbitals split into two sets of two and three orbitals. In octahedral geometry of the complex, ligand approach from the axis and the orbitals ${{\text{d}}_{{{\text{x}}^{\text{2}}} - {{\text{y}}^{\text{2}}}}}$ and ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$ are present on the axis whereas${{\text{d}}_{{\text{xy}}}}$,${{\text{d}}_{{\text{zy}}}}$ and ${{\text{d}}_{{\text{xz}}}}$ orbitals lie in between the axes. So, the energy of two orbitals ${{\text{d}}_{{{\text{x}}^{\text{2}}} - {{\text{y}}^{\text{2}}}}}$ and ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$ increases due to the ligands and the energy of three orbitals decreases. As a result, five degenerate-orbitals lose that degeneracy and split into two groups.
The CFT diagram of the ${\text{S}}{{\text{c}}^{{\text{3 + }}}}$and ${\text{C}}{{\text{r}}^{{\text{3 + }}}}$is as follows:


From the CFT diagram, we can say that the ${\text{S}}{{\text{c}}^{{\text{3 + }}}}$ has no d-electron so, no d-d transition is possible so, the ${\text{S}}{{\text{c}}^{{\text{3 + }}}}$is colourless whereas the ${\text{C}}{{\text{r}}^{{\text{3 + }}}}$has three unpaired electrons so, the d-d transition is possible so, the ${\text{C}}{{\text{r}}^{{\text{3 + }}}}$is coloured.
Note: Colour of $3{\text{d}} - $transition metals depend upon the d-d-transition and number of unpaired electrons in d-orbitals. Transmitted colour appears as colour of substance due to the presence of electrons that are responsible for transition. The presence of unpaired electrons also tells the magnetic nature of the complex. The given titanium complex is paramagnetic due to the presence of one unpaired electron. If all the electrons are paired then the complex will be diamagnetic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




