
-What is the shape of chromate ion? Draw its structure.
Answer
585.9k+ views
Hint:At first think about the chemical formula and molecular geometry of chromate ions. The structure of a chemical compound determines the molecular geometry of a compound by the spatial arrangement of atoms and chemical bonds in the molecule.
Complete step by step answer:
-According to the chemical formula of chromate ion, its structure is
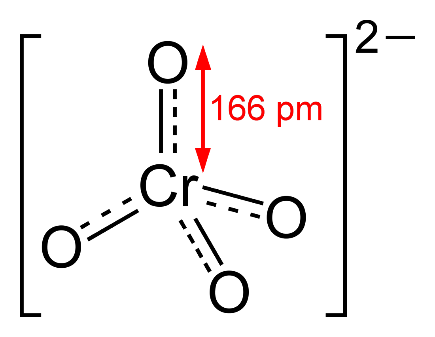
The shape of \[Cr{O_4}^{ - 2}\] is tetrahedral.
Additional Information:-Chromate ion is an oxoanion of chromium in the $ + 6$ oxidation state. It is a moderately strong oxidizing agent. In an aqueous solution chromate and dichromate ions can be inter convertible. Chromates are used in chrome plating to protect metals from corrosion and to improve paint adhesion. Chromate salts of heavy metals, lanthanides and alkaline earth metals are very slightly soluble in water and used as pigments. The lead containing pigment chrome yellow was used for a very long time. The primary chromium ore is the mixed metal oxide chromite $FeC{r_2}{O_4}$ and found as brittle metallic black crystals or granules. Chromite ore is heated with a mixture of calcium carbonate and sodium carbonate in the presence of air. All hexavalent compounds of chromium are toxic and carcinogenic, especially if inhaled they cause lung cancer. The use of chromate compounds in manufactured goods is restricted.
Note:
-Don’t get confused with the molecular geometry and the representation of structure of a chemical compound. Molecular geometry is determined by the quantum mechanical behavior of electrons. Structure of a chemical compound is based on spatial arrangement of atoms and chemical bonds.
Complete step by step answer:
-According to the chemical formula of chromate ion, its structure is

The shape of \[Cr{O_4}^{ - 2}\] is tetrahedral.
Additional Information:-Chromate ion is an oxoanion of chromium in the $ + 6$ oxidation state. It is a moderately strong oxidizing agent. In an aqueous solution chromate and dichromate ions can be inter convertible. Chromates are used in chrome plating to protect metals from corrosion and to improve paint adhesion. Chromate salts of heavy metals, lanthanides and alkaline earth metals are very slightly soluble in water and used as pigments. The lead containing pigment chrome yellow was used for a very long time. The primary chromium ore is the mixed metal oxide chromite $FeC{r_2}{O_4}$ and found as brittle metallic black crystals or granules. Chromite ore is heated with a mixture of calcium carbonate and sodium carbonate in the presence of air. All hexavalent compounds of chromium are toxic and carcinogenic, especially if inhaled they cause lung cancer. The use of chromate compounds in manufactured goods is restricted.
Note:
-Don’t get confused with the molecular geometry and the representation of structure of a chemical compound. Molecular geometry is determined by the quantum mechanical behavior of electrons. Structure of a chemical compound is based on spatial arrangement of atoms and chemical bonds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




