
What is the shape of $ ClF_2^ - $ ion?
(A) Bent
(B) Linear
(C) Pyramidal
(D) None of these
Answer
550.8k+ views
Hint: To find the shape of a molecule, we need to identify the Lewis structure of the ion, and using the Lewis structure find the Steric number. Then using the VSEPR theory we can infer the shape, and hybridisation of the molecular structure of the given compound from its steric number.
We also know that the Steric number is given by, SN= (number of lone pair of electrons on the central atom) + (number of atoms bonded to the central atom)
Formulas used: We will be using the formula to find the steric number of a molecular structure that is given by, SN= (number of lone pairs of electrons on the central atom) + (number of atoms bonded to the central atom).
Complete Step by Step answer
We know that both fluorine and chlorine are elements of the halogen group from the periodic table. They are monovalent ions with 7 valence electrons in its outermost orbit. Since all the three constituting elements are halogens with similar valency, the heaviest and the element with least electronegativity will be the central atom.
Hence in this case, $ Cl $ is the central atom while the fluorine atoms form single bonds (due to its mono-valency). Drawing the Lewis structure, we get,
Valence electrons on chlorine and fluorine =7 so, the total number of electrons on the given ion will be, $ 7 + 7\left( 2 \right) + 1 = 22 $ .
Thus, the Lewis structure will be,
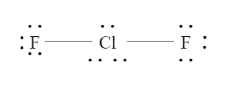
We know that each element forming a bond must have an octet electron exactly. Here we can see that the chlorine has more electrons than an octet, this is because the halogens can expand their octet while bonding among themselves.
Now we can see that the central atom Chlorine has 3 lone pairs of electrons while it is bonded to two other fluorine atoms. Steric number, SN= 3+2=5.
We now know from the Lewis structure the steric number of the ion given is 5. Using the VSEPR theory we can find the hybridisation of the ion to be, $ s{p^3}d $ . From the hybridisation structures of $ s{p^3}d $ we know if a compound has 3 lone pairs and 2 bond pairs then it forms a linear structure with $ 180^\circ $ .
Note
Alternate method: We know that the central atom will be chlorine and it holds 7 valence electrons and also holds an extra electron, hence 8 electrons. We also know that the 2 fluorine atoms bond by occupying an electron each, thus leaving you with 2 bonded and 3 lone pairs on the central atom. Thus, forming a $ s{p^3}d $ hybridised structure. Again, since it has 3 lone and 2 bond pairs the molecule will have a linear structure.
We also know that the Steric number is given by, SN= (number of lone pair of electrons on the central atom) + (number of atoms bonded to the central atom)
Formulas used: We will be using the formula to find the steric number of a molecular structure that is given by, SN= (number of lone pairs of electrons on the central atom) + (number of atoms bonded to the central atom).
Complete Step by Step answer
We know that both fluorine and chlorine are elements of the halogen group from the periodic table. They are monovalent ions with 7 valence electrons in its outermost orbit. Since all the three constituting elements are halogens with similar valency, the heaviest and the element with least electronegativity will be the central atom.
Hence in this case, $ Cl $ is the central atom while the fluorine atoms form single bonds (due to its mono-valency). Drawing the Lewis structure, we get,
Valence electrons on chlorine and fluorine =7 so, the total number of electrons on the given ion will be, $ 7 + 7\left( 2 \right) + 1 = 22 $ .
Thus, the Lewis structure will be,

We know that each element forming a bond must have an octet electron exactly. Here we can see that the chlorine has more electrons than an octet, this is because the halogens can expand their octet while bonding among themselves.
Now we can see that the central atom Chlorine has 3 lone pairs of electrons while it is bonded to two other fluorine atoms. Steric number, SN= 3+2=5.
We now know from the Lewis structure the steric number of the ion given is 5. Using the VSEPR theory we can find the hybridisation of the ion to be, $ s{p^3}d $ . From the hybridisation structures of $ s{p^3}d $ we know if a compound has 3 lone pairs and 2 bond pairs then it forms a linear structure with $ 180^\circ $ .
Note
Alternate method: We know that the central atom will be chlorine and it holds 7 valence electrons and also holds an extra electron, hence 8 electrons. We also know that the 2 fluorine atoms bond by occupying an electron each, thus leaving you with 2 bonded and 3 lone pairs on the central atom. Thus, forming a $ s{p^3}d $ hybridised structure. Again, since it has 3 lone and 2 bond pairs the molecule will have a linear structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




