
Shape of P-orbital is:
A. Spherical
B. dumb-Bell
C. Double dumb bell
D. None of these
Answer
567.3k+ views
Hint:To determine the shape of p-orbitals we should know the structure of the p-orbital and the number of lobes present in the p-orbital. P- subshell has three orbits. Three orbital shells are denoted as ${{\text{P}}_{\text{X}}}$,${{\text{P}}_{\text{Y}}}$, and ${{\text{P}}_{\text{Z}}}$.
Complete step-by-step solution:The number of orbitals in a subshell is determined by the following formula:
${\text{2l}}\,{\text{ + }}\,{\text{1}}$
Where,
${\text{l}}\,$ is the azimuthal quantum number.
The l value of p-orbital is $1$.
So,
${\text{2}} \times {\text{1}}\,{\text{ + }}\,{\text{1}}$
$3$
The P-subshell has three, ${{\text{P}}_{\text{X}}}$,${{\text{P}}_{\text{Y}}}$and ${{\text{P}}_{\text{Z}}}$orbitals. All these orbitals are degenerate. All orbitals of the p-subshell lie on the axis. So, the electron density of each orbital also lies on the axis. Each p-orbital has two lobes.
The positions of p-orbitals on the axis is represented as follows:
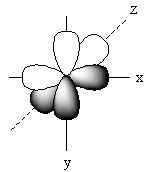
The positions of these three p-orbitals on the axis separately is represented as follows:
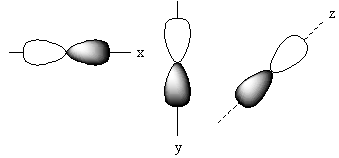
Name of each p-orbital depends upon the axis. The p-orbital which lies on the x-axis is known as ${{\text{P}}_{\text{x}}}$ orbital. The p-orbital which lies on the y-axis is known as ${{\text{P}}_{\text{Y}}}$ orbital. The p-orbital which lies on the z-axis is known as ${{\text{P}}_{\text{Z}}}$ orbital.
Each p-orbital has two lobes that are arranged like a dumb bell so, the shape of p–orbital is dumb bell.
So, the shape of P-orbital is dumb-Bell.
Therefore, option (B) dumb-Bell, is correct.
Note:The s-subshell has only one s-orbital. S-orbital has one lobe of spherical shape so, the shape of s-orbital is spherical. D-subshell has five d-orbitals. Out of five d-orbitals, two lie on the axis and three lie in between the axis. ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$and ${{\text{d}}_{{{\text{X}}^2} - {{\text{Y}}^{\text{2}}}}}$orbitals which lies on the axis and the ${{\text{d}}_{XZ}}$,${{\text{d}}_{YZ}}$, and ${{\text{d}}_{XY}}$ lies in between the axis. Except ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$all d-orbital has four lobes so, the shape of d-orbital is double dumb bell.
Complete step-by-step solution:The number of orbitals in a subshell is determined by the following formula:
${\text{2l}}\,{\text{ + }}\,{\text{1}}$
Where,
${\text{l}}\,$ is the azimuthal quantum number.
The l value of p-orbital is $1$.
So,
${\text{2}} \times {\text{1}}\,{\text{ + }}\,{\text{1}}$
$3$
The P-subshell has three, ${{\text{P}}_{\text{X}}}$,${{\text{P}}_{\text{Y}}}$and ${{\text{P}}_{\text{Z}}}$orbitals. All these orbitals are degenerate. All orbitals of the p-subshell lie on the axis. So, the electron density of each orbital also lies on the axis. Each p-orbital has two lobes.
The positions of p-orbitals on the axis is represented as follows:

The positions of these three p-orbitals on the axis separately is represented as follows:

Name of each p-orbital depends upon the axis. The p-orbital which lies on the x-axis is known as ${{\text{P}}_{\text{x}}}$ orbital. The p-orbital which lies on the y-axis is known as ${{\text{P}}_{\text{Y}}}$ orbital. The p-orbital which lies on the z-axis is known as ${{\text{P}}_{\text{Z}}}$ orbital.
Each p-orbital has two lobes that are arranged like a dumb bell so, the shape of p–orbital is dumb bell.
So, the shape of P-orbital is dumb-Bell.
Therefore, option (B) dumb-Bell, is correct.
Note:The s-subshell has only one s-orbital. S-orbital has one lobe of spherical shape so, the shape of s-orbital is spherical. D-subshell has five d-orbitals. Out of five d-orbitals, two lie on the axis and three lie in between the axis. ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$and ${{\text{d}}_{{{\text{X}}^2} - {{\text{Y}}^{\text{2}}}}}$orbitals which lies on the axis and the ${{\text{d}}_{XZ}}$,${{\text{d}}_{YZ}}$, and ${{\text{d}}_{XY}}$ lies in between the axis. Except ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$all d-orbital has four lobes so, the shape of d-orbital is double dumb bell.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




