
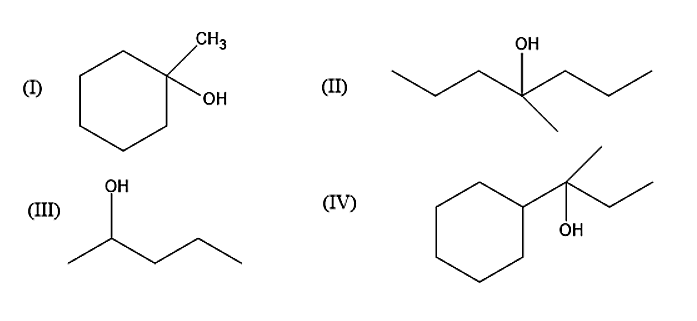
Show how would you synthesis the following alcohols from appropriate alkenes?

Answer
565.8k+ views
Hint: In order to answer the given question, we have to remember that the main chemical reactions which alkenes will be undergoing are the addition reactions. The addition reaction will convert the carbon-carbon double bond of alkenes into the carbon-carbon single bond when some other external functional group is added.
Complete Solution :
Let us first understand the addition reaction. Addition reaction is a chemical reaction which involves the conversion of the carbon-carbon double bond of alkenes into the carbon-carbon single bond when some other external functional group is added.
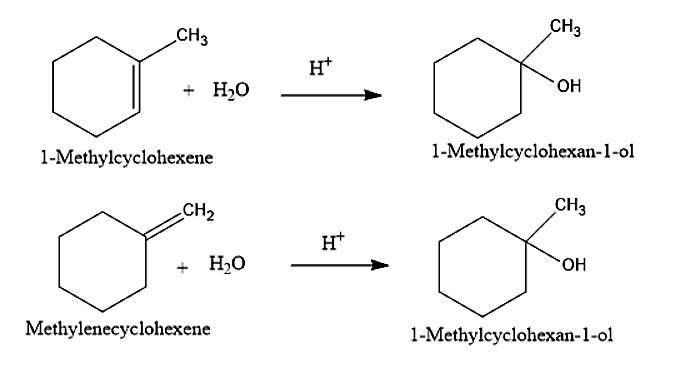
(I) Now let us move onto the question given. The first alcohol given is 1-Methylcyclohexan-1-ol. It can be prepared from two alkenes namely 1-Methylcyclohexene and Methylenecyclohexene. The addition of water molecules to these two alkenes will give 1-Methylcyclohexan-1-ol. When the water is added the Carbon-carbon double bond present in the alkenes will be converted into carbon-carbon single bond.
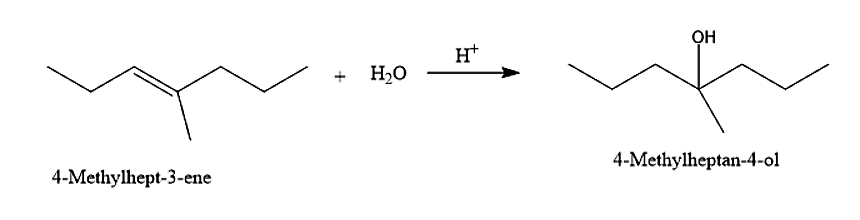
(II) The second alcohol given is the 4-Methyl cycloheptane-4-ol. The 4-Methyl cycloheptane-4-ol can be prepared from 4-Methyl hept-3-ene by the addition of water molecules.
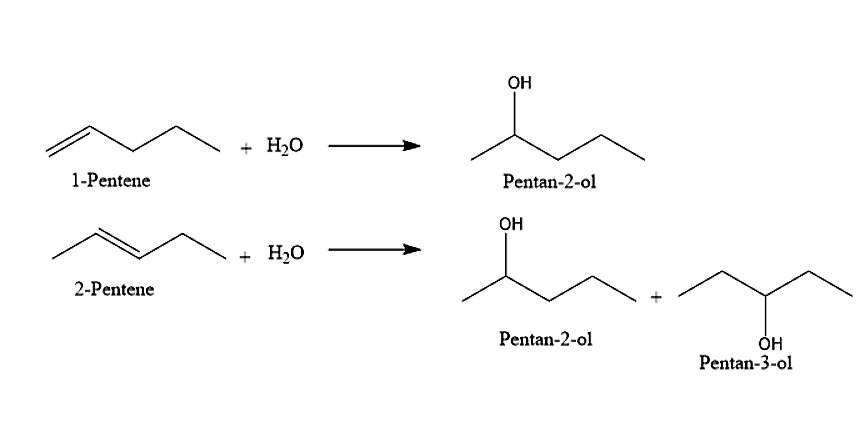
(III) The third alcohol given is the pentan-2-ol. It can be obtained from two alkenes namely 1-Pentene and 2-Pentene. Addition of water molecules to these two alkenes will give Pentan-2-ol.
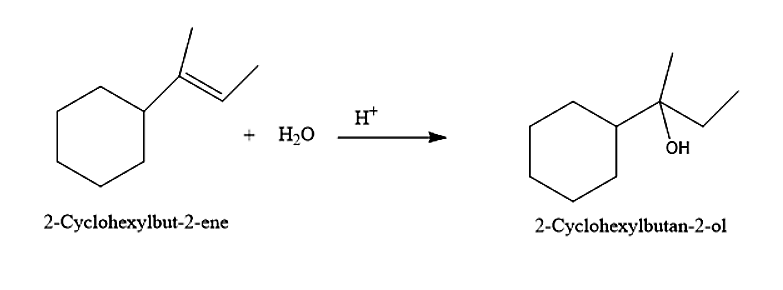
(IV) The fourth alcohol given is 2-Cyclohexyl Butan-2-ol. This alcohol can be prepared by the addition of water molecules to 2-Cyclohexyl But-2-ene.
Note: The other addition reaction which can take place in the alkene molecule are the following:
- Addition of hydrogen
- Addition of Halogen
- Addition of Hydrogen halide
Complete Solution :
Let us first understand the addition reaction. Addition reaction is a chemical reaction which involves the conversion of the carbon-carbon double bond of alkenes into the carbon-carbon single bond when some other external functional group is added.
(I) Now let us move onto the question given. The first alcohol given is 1-Methylcyclohexan-1-ol. It can be prepared from two alkenes namely 1-Methylcyclohexene and Methylenecyclohexene. The addition of water molecules to these two alkenes will give 1-Methylcyclohexan-1-ol. When the water is added the Carbon-carbon double bond present in the alkenes will be converted into carbon-carbon single bond.

(II) The second alcohol given is the 4-Methyl cycloheptane-4-ol. The 4-Methyl cycloheptane-4-ol can be prepared from 4-Methyl hept-3-ene by the addition of water molecules.

(III) The third alcohol given is the pentan-2-ol. It can be obtained from two alkenes namely 1-Pentene and 2-Pentene. Addition of water molecules to these two alkenes will give Pentan-2-ol.

(IV) The fourth alcohol given is 2-Cyclohexyl Butan-2-ol. This alcohol can be prepared by the addition of water molecules to 2-Cyclohexyl But-2-ene.

Note: The other addition reaction which can take place in the alkene molecule are the following:
- Addition of hydrogen
- Addition of Halogen
- Addition of Hydrogen halide
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




