
How many sigma and pi bonds are present in tetra cyano methane?
Answer
538.5k+ views
Hint:The total number of bonds in any compound is the sum of total sigma bonds and pi bonds. The molecular formula of tetra cyano methane is \[C{(CN)_4}\].
Complete step-by-step answer:Tetra cyano methane is also known as carbon tetracyanide. It is a per cyano alkane with molecular formula \[C{(CN)_4}\]. The structure can be considered as methane with all hydrogen atoms replaced by cyanide groups. Tetra cyano methane is a solid at room temperature. It decomposes over 160 °C without melting, and although it can be in a dilute vapour, no liquid form is known.
The molecules of tetra cyano methane have a tetrahedral geometry with carbon atoms at the centre and four CN atoms surrounding it. The C-C bond length is 1.484 and the C≡N bond length is 1.161 in the gas form. In the solid the C≡N bond shortens to 1.147. At pressures over 7 GPa tetra cyano methane starts to polymerize to form a disorganised covalent network solid. At higher pressure the colour yellows and darkens to black.
The structure of tetra cyano methane is:
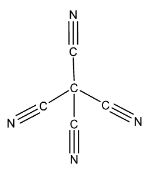
As clearly seen from the structure, there are four C-C sigma bonds and four C-N sigma bonds. So, there are a total of eight sigma bonds. And there are eight C-N pi bonds. So, there are a total of eight pi bonds. Hence, there are a total of 16 bonds in tetra cyano methane.
Note:When asked about the total bonds of \[C{(CN)_4}\], a student can forget to count the bonds between carbon and nitrogen. Then his incorrect answer would come out to be 4.
Complete step-by-step answer:Tetra cyano methane is also known as carbon tetracyanide. It is a per cyano alkane with molecular formula \[C{(CN)_4}\]. The structure can be considered as methane with all hydrogen atoms replaced by cyanide groups. Tetra cyano methane is a solid at room temperature. It decomposes over 160 °C without melting, and although it can be in a dilute vapour, no liquid form is known.
The molecules of tetra cyano methane have a tetrahedral geometry with carbon atoms at the centre and four CN atoms surrounding it. The C-C bond length is 1.484 and the C≡N bond length is 1.161 in the gas form. In the solid the C≡N bond shortens to 1.147. At pressures over 7 GPa tetra cyano methane starts to polymerize to form a disorganised covalent network solid. At higher pressure the colour yellows and darkens to black.
The structure of tetra cyano methane is:

As clearly seen from the structure, there are four C-C sigma bonds and four C-N sigma bonds. So, there are a total of eight sigma bonds. And there are eight C-N pi bonds. So, there are a total of eight pi bonds. Hence, there are a total of 16 bonds in tetra cyano methane.
Note:When asked about the total bonds of \[C{(CN)_4}\], a student can forget to count the bonds between carbon and nitrogen. Then his incorrect answer would come out to be 4.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




