
How many $\sigma $ and $\pi $ bonds are present in the structure of D.D.T respectively?
(a)- 21, 6
(b)- 17, 6
(c)- 20, 6
(d)- 29, 6
Answer
568.2k+ views
Hint: If a compound has carbon atoms, then there are two types of bonds: sigma and pi bond. The single bond represents the single bond and the double and triple bond represents the pi bond. Two electrons are involved in the formation of a bond. The bond of carbon and other atoms depends on the valency.
Complete Solution :
- In an organic compound, there are mostly carbon atoms present and these carbon atoms are mostly connected with hydrogen, oxygen, nitrogen, chlorine, etc. So, the bonds are mostly made up of two bonds: a sigma bond and a pi bond. The compound is having only a sigma bond if the compound has a single bond. If the compound has one sigma bond and one pi bond, then the compound is having a double bond. If the compound has one sigma bond and two pi bonds, then the compound is having a triple bond. The sigma electrons are the electrons that form the sigma bond and pi electrons are the electrons that form the pi bond.
So, the compound given in the question is D.D.T. (p, p’-DichlorodiphenylTrichloroethane) whose formula is ${{C}_{14}}{{H}_{9}}C{{l}_{5}}$ and its structure is given below:
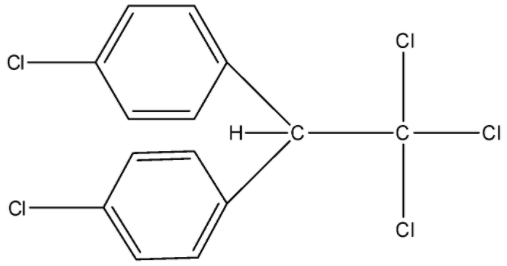
So, in the structure, there are a total of 27 bonds in which 6 are the double bonds and the rest 21 bonds are the single bond. Therefore, there are 21 $\sigma $ bonds and 6 $\pi $ bonds.
So, the correct answer is “Option A”.
Note: If the compounds have two sigma electrons and four pi electrons then the compound has a triple bond. These bonds are only formed if the compound has a covalent bond or we can say that both the sigma and pi bonds are covalent.
Complete Solution :
- In an organic compound, there are mostly carbon atoms present and these carbon atoms are mostly connected with hydrogen, oxygen, nitrogen, chlorine, etc. So, the bonds are mostly made up of two bonds: a sigma bond and a pi bond. The compound is having only a sigma bond if the compound has a single bond. If the compound has one sigma bond and one pi bond, then the compound is having a double bond. If the compound has one sigma bond and two pi bonds, then the compound is having a triple bond. The sigma electrons are the electrons that form the sigma bond and pi electrons are the electrons that form the pi bond.
So, the compound given in the question is D.D.T. (p, p’-DichlorodiphenylTrichloroethane) whose formula is ${{C}_{14}}{{H}_{9}}C{{l}_{5}}$ and its structure is given below:

So, in the structure, there are a total of 27 bonds in which 6 are the double bonds and the rest 21 bonds are the single bond. Therefore, there are 21 $\sigma $ bonds and 6 $\pi $ bonds.
So, the correct answer is “Option A”.
Note: If the compounds have two sigma electrons and four pi electrons then the compound has a triple bond. These bonds are only formed if the compound has a covalent bond or we can say that both the sigma and pi bonds are covalent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




