
How many sigma/pi bonds are in the aspirin structure?
Answer
559.2k+ views
Hint First of all, you should know the structure of the aspirin and then, identify the number of single bonds i.e. sigma bonds present in it and the number of double bonds i.e. pi bonds present in it , you will get your answer. Now solve it.
Complete answer:
First of all, let’s discuss what sigma and pi bonds are. Sigma bonds are those which are formed by the end to end overlapping of the two half -filed atomic orbitals along the internuclear-axis.
On the other hand, pi bonds are those which are formed by the sidewise or the lateral overlapping of the two atomic orbitals and the sidewise overlapping can take place only when the orbitals have their lobes perpendicular to the molecular axis.
Now considering the statement as;
The structure of aspirin is as follows;
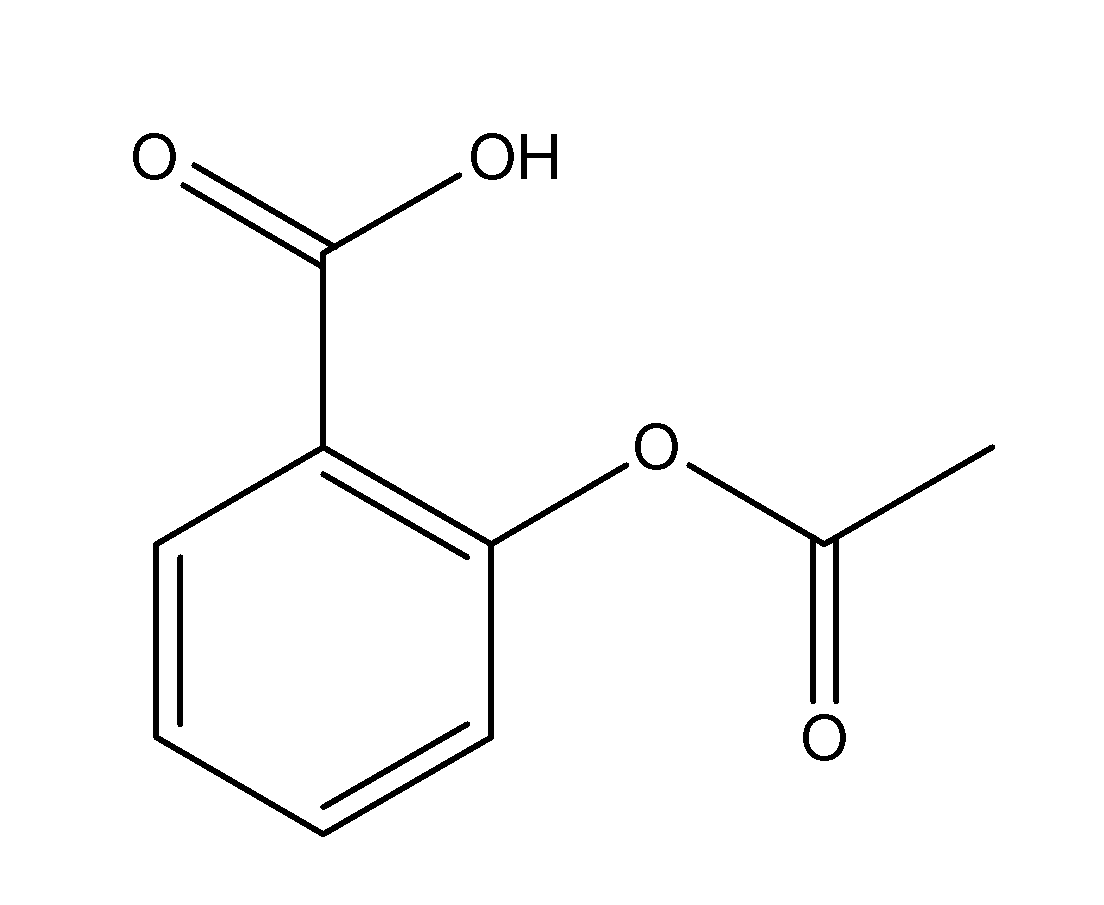
From the structure, we can see that the number of single bonds i.e. sigma bonds is equal to 13 ( 5 C-O bonds, 2 C-C bonds outside the benzene ring and 6 C-C bonds within the benzene ring)
And the number of double bonds i.e. pi bonds is equal to 5 ( 2 C-O pi-bonds outside the ring and 3 C-C pi bonds inside the benzene ring.
Thus, the number of sigma and pi bonds present in the structure of aspirin are 13 and 5 respectively.
Note: In case of the sigma bond, the orbitals overlap end to end and the extent of overlapping is large as compared to the sidewise overlapping in case of the pi-bonds. So, therefore, sigma bond is a strong bond and pi-bond is a weak bond.
Complete answer:
First of all, let’s discuss what sigma and pi bonds are. Sigma bonds are those which are formed by the end to end overlapping of the two half -filed atomic orbitals along the internuclear-axis.
On the other hand, pi bonds are those which are formed by the sidewise or the lateral overlapping of the two atomic orbitals and the sidewise overlapping can take place only when the orbitals have their lobes perpendicular to the molecular axis.
Now considering the statement as;
The structure of aspirin is as follows;

From the structure, we can see that the number of single bonds i.e. sigma bonds is equal to 13 ( 5 C-O bonds, 2 C-C bonds outside the benzene ring and 6 C-C bonds within the benzene ring)
And the number of double bonds i.e. pi bonds is equal to 5 ( 2 C-O pi-bonds outside the ring and 3 C-C pi bonds inside the benzene ring.
Thus, the number of sigma and pi bonds present in the structure of aspirin are 13 and 5 respectively.
Note: In case of the sigma bond, the orbitals overlap end to end and the extent of overlapping is large as compared to the sidewise overlapping in case of the pi-bonds. So, therefore, sigma bond is a strong bond and pi-bond is a weak bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




