
Sodium benzene sulphonate after reaction with $NaOH$ undergoes acidic hydrolysis, the compound formed would be:
(a)- Phenol
(b)- Benzoic acid
(c)- Benzene
(d)- Disodium benzaldehyde
Answer
570k+ views
Hint: Sodium benzene sulphonate is an aromatic compound in which the carbon atom of the benzene is attached to $-S{{O}_{3}}Na$ group. When the sodium benzene sulphonate is treated with sodium hydroxide, the by-products are sodium sulfite and water.
Complete Solution :
Sodium benzene sulphonate is an aromatic compound in which the carbon atom of the benzene is attached to $-S{{O}_{3}}Na$ group. The structure of sodium benzene sulphonate is given below:
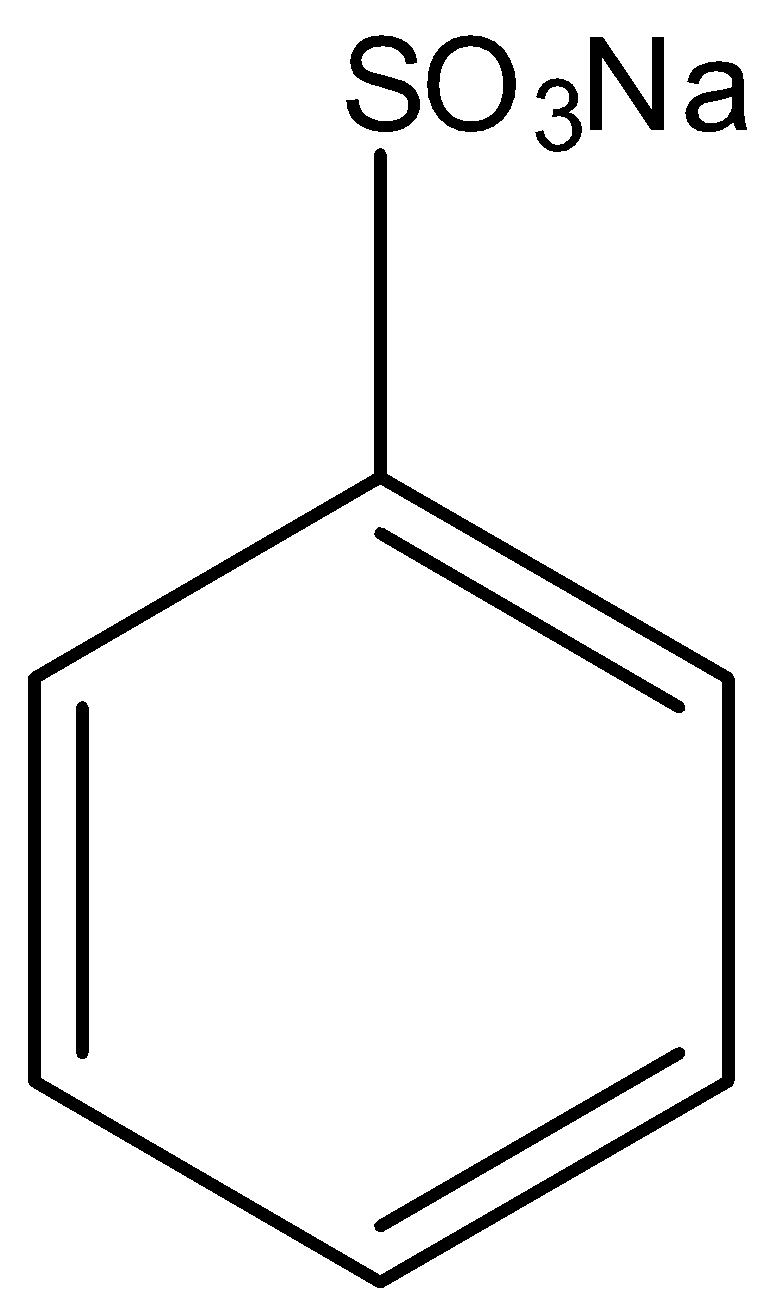
When the sodium benzene sulphonate is reacted with sodium hydroxide, the sodium sulfonate group from the benzene is replaced with the sodium oxide group, and there is the formation of sodium phenoxide and the by-products are sodium sulfite and water. Now when this sodium phenoxide is hydrolyzed means the sodium phenoxide is reacted with water, the sodium ion in the sodium phenoxide is replaced with a hydrogen ion, so there is formation of phenol. And the by-product of this reaction is sodium hydroxide. This reaction is the acidification of sodium phenoxide forming phenol. This reaction can only occur in the presence of acid. The two-step reaction is given below:
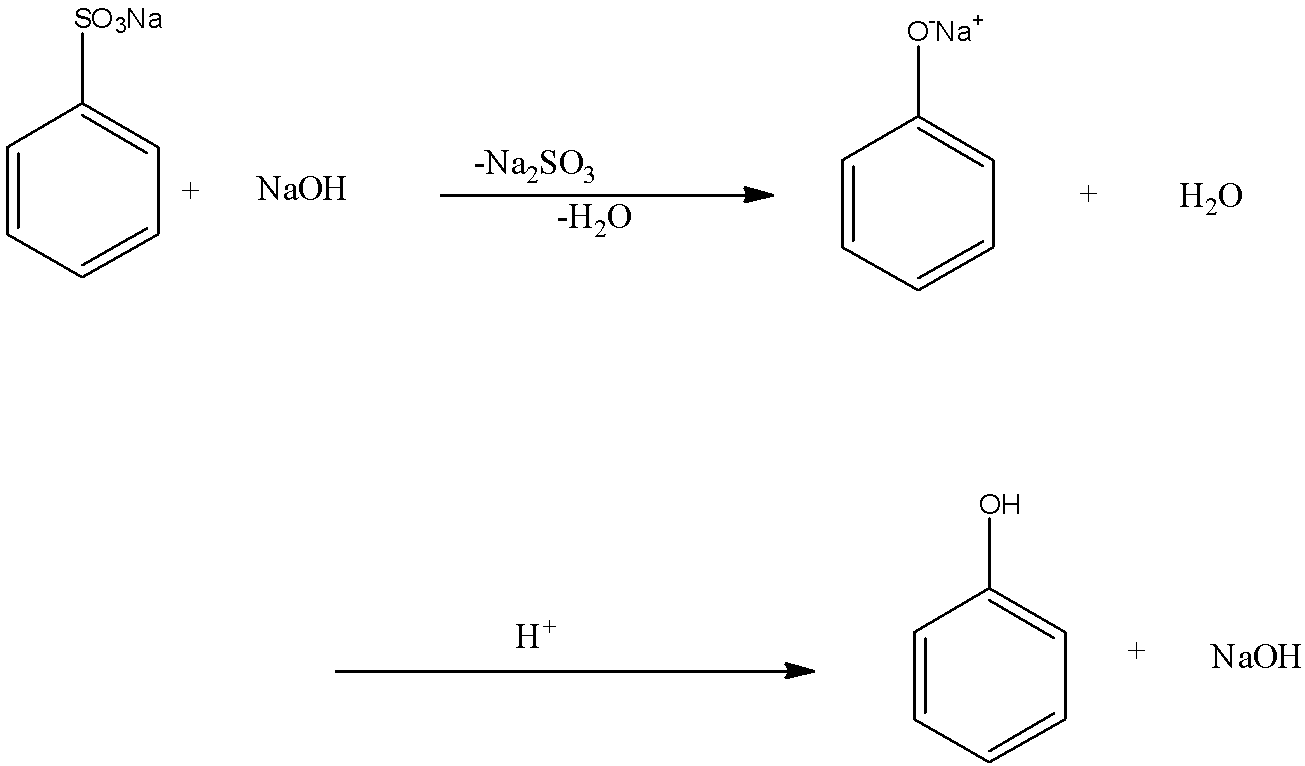
So the final product in this reaction is phenol. Therefore, the correct answer is an option (a)- Phenol.
So, the correct answer is “Option A”.
Note: This is one main process for the production of phenol and this process is mostly followed in the laboratory. The temperature for this reaction is 573 – 623 K. The sodium benzene sulphonate can be easily produced from benzene sulphonic acid.
Complete Solution :
Sodium benzene sulphonate is an aromatic compound in which the carbon atom of the benzene is attached to $-S{{O}_{3}}Na$ group. The structure of sodium benzene sulphonate is given below:

When the sodium benzene sulphonate is reacted with sodium hydroxide, the sodium sulfonate group from the benzene is replaced with the sodium oxide group, and there is the formation of sodium phenoxide and the by-products are sodium sulfite and water. Now when this sodium phenoxide is hydrolyzed means the sodium phenoxide is reacted with water, the sodium ion in the sodium phenoxide is replaced with a hydrogen ion, so there is formation of phenol. And the by-product of this reaction is sodium hydroxide. This reaction is the acidification of sodium phenoxide forming phenol. This reaction can only occur in the presence of acid. The two-step reaction is given below:

So the final product in this reaction is phenol. Therefore, the correct answer is an option (a)- Phenol.
So, the correct answer is “Option A”.
Note: This is one main process for the production of phenol and this process is mostly followed in the laboratory. The temperature for this reaction is 573 – 623 K. The sodium benzene sulphonate can be easily produced from benzene sulphonic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




