
Sodium chloride is an ionic compound whereas hydrogen chloride is mainly covalent because_________.
A. sodium is less reactive
B. hydrogen is non-metal
C. hydrogen chloride is a gas
D. electronegativity difference in the case of Hydrogen and chlorine is less than 21
Answer
558.3k+ views
Hint: The type bonding between atoms is dependent on the type of the combining atoms. If the bond is formed between a metal and non-metal, that type of bond is an ionic bond and if two non-metals are combined, then the type of bond formed between the nonmetals is covalent.
Complete step by step answer:
Let’s discuss the type of bonding namely, ionic and covalent bonding in detail.
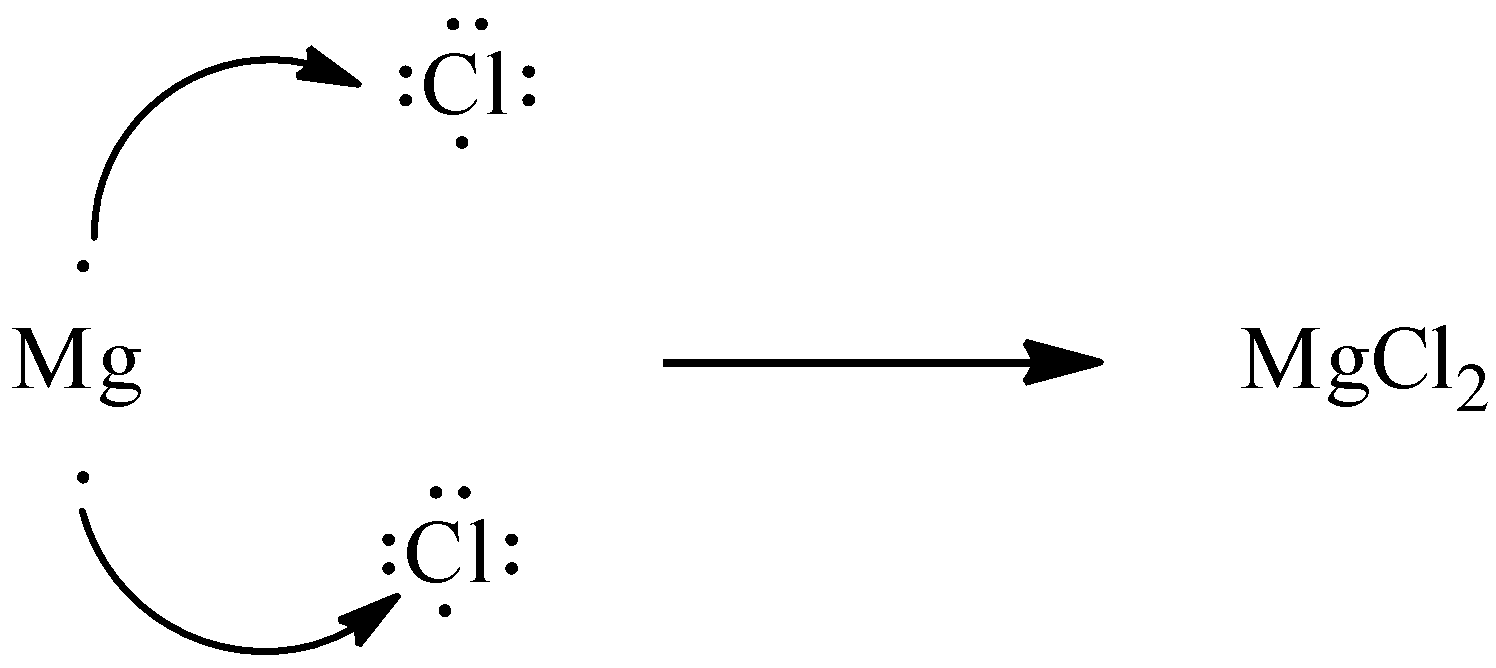
Ionic bonding forms when one metal and one non-metal undergo combination. Let’s take the example of ${\rm{MgC}}{{\rm{l}}_{\rm{2}}}$. In ${\rm{MgC}}{{\rm{l}}_{\rm{2}}}$, Magnesium is metal and chlorine is non-metal.
Let’s discuss the formation of ${\rm{MgC}}{{\rm{l}}_{\rm{2}}}$.
Magnesium is the element whose atomic number is 12. So, its electronic configuration is 2,8,2. Chlorine is a non-metal whose atomic number is 17, that means, electronic configuration is 2,8,7.
So, from the above electronic configurations of magnesium and chlorine, we get to know that magnesium can lose two electrons and chlorine can accept one electron to achieve noble gas configuration. As magnesium loses two electrons, we require two chlorine atoms to accept the two electrons.
Let’s discuss covalent bonding in detail. It is the bonding that forms when two nonmetals combine. In this bonding electron is shared between the atoms. For example, ${\rm{C}}{{\rm{H}}_{\rm{4}}}$. In ${\rm{C}}{{\rm{H}}_{\rm{4}}}$, carbon and hydrogen both are non-metal.
The atomic number of hydrogen is 1 (electronic configuration is 1). The atomic number of carbon is 6, that means, electronic configuration is 2,4. Now, carbon needed 4 electrons and hydrogen needed two electrons to achieve stable configuration. So, the carbon atom shares its four electrons with four hydrogen atoms and hydrogen atoms share its electrons with the carbon atom.
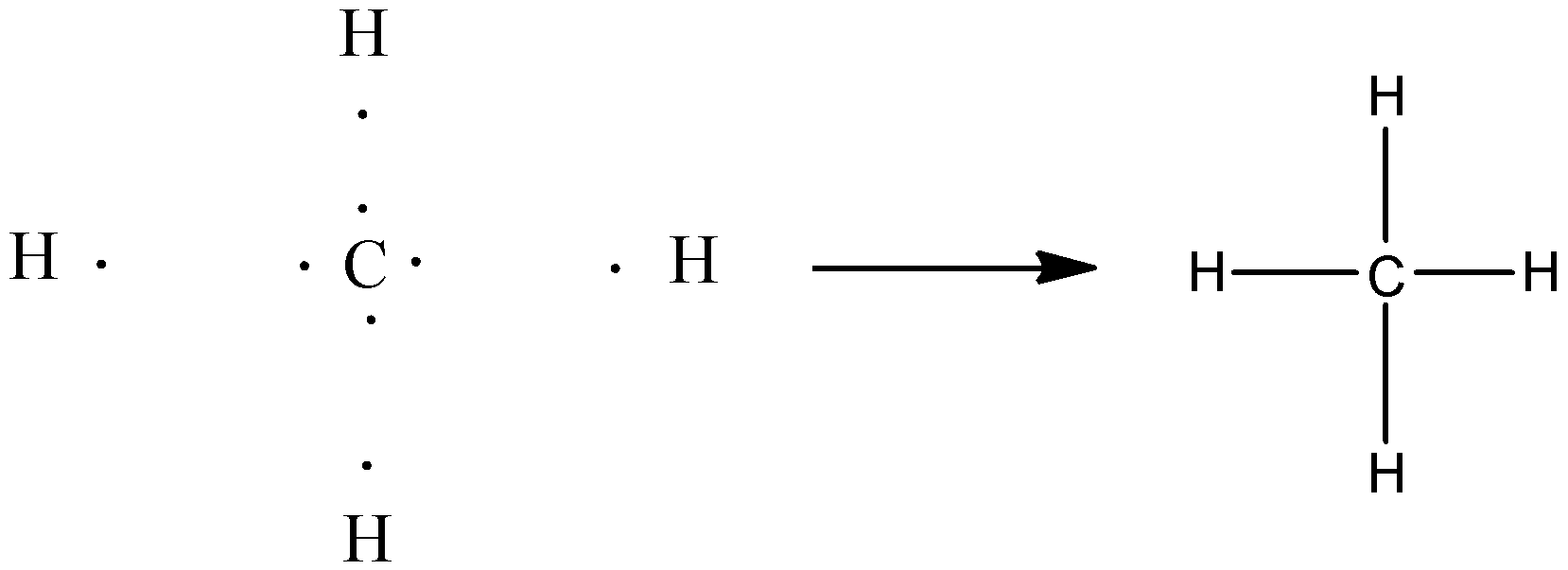
Now, both the elements achieve octet and thus a covalent compound forms.
Now, come to the question. Sodium chloride is an ionic compound as sodium is a metal and chlorine is a non-metal but hydrogen chlorine is a covalent compound as hydrogen and chlorine both are non metals.
Hence, the correct answer is option B.
Note:
Always remember that in an ionic compound, one element is electronegative and the other element is electronegative. By the term ‘electropositive’ we mean that an element can lose electrons (metals) and electronegative means that an element can gain electrons (non-metals).
Complete step by step answer:
Let’s discuss the type of bonding namely, ionic and covalent bonding in detail.
Ionic bonding forms when one metal and one non-metal undergo combination. Let’s take the example of ${\rm{MgC}}{{\rm{l}}_{\rm{2}}}$. In ${\rm{MgC}}{{\rm{l}}_{\rm{2}}}$, Magnesium is metal and chlorine is non-metal.
Let’s discuss the formation of ${\rm{MgC}}{{\rm{l}}_{\rm{2}}}$.
Magnesium is the element whose atomic number is 12. So, its electronic configuration is 2,8,2. Chlorine is a non-metal whose atomic number is 17, that means, electronic configuration is 2,8,7.
So, from the above electronic configurations of magnesium and chlorine, we get to know that magnesium can lose two electrons and chlorine can accept one electron to achieve noble gas configuration. As magnesium loses two electrons, we require two chlorine atoms to accept the two electrons.

Let’s discuss covalent bonding in detail. It is the bonding that forms when two nonmetals combine. In this bonding electron is shared between the atoms. For example, ${\rm{C}}{{\rm{H}}_{\rm{4}}}$. In ${\rm{C}}{{\rm{H}}_{\rm{4}}}$, carbon and hydrogen both are non-metal.
The atomic number of hydrogen is 1 (electronic configuration is 1). The atomic number of carbon is 6, that means, electronic configuration is 2,4. Now, carbon needed 4 electrons and hydrogen needed two electrons to achieve stable configuration. So, the carbon atom shares its four electrons with four hydrogen atoms and hydrogen atoms share its electrons with the carbon atom.

Now, both the elements achieve octet and thus a covalent compound forms.
Now, come to the question. Sodium chloride is an ionic compound as sodium is a metal and chlorine is a non-metal but hydrogen chlorine is a covalent compound as hydrogen and chlorine both are non metals.
Hence, the correct answer is option B.
Note:
Always remember that in an ionic compound, one element is electronegative and the other element is electronegative. By the term ‘electropositive’ we mean that an element can lose electrons (metals) and electronegative means that an element can gain electrons (non-metals).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




