
Sodium chloride is used in the soap industry for soap’s ______.
(A) decomposition
(B) removal of hardness
(C) precipitation
(D) none of the above
Answer
586.2k+ views
Hint: Recollect the process of commercial manufacturing of soap. Make a note of all the reagents used in the process and the reason for using them. Find out for which process table salt is used to get the answer.
Complete step by step solution:
- The process of manufacturing of soap is known as saponification.
- Soaps are sodium or potassium salts of long chains of fatty acids which contain more than 12 carbon atoms.
- In saponification, fats or oils are first hydrolysed by using sodium hydroxide, NaOH or potassium hydroxide, KOH to form a solution of glycerol and fatty acids.
- Soap is then precipitated from the solution by adding salt like sodium chloride, NaCl, potassium chloride, KCl, etc.
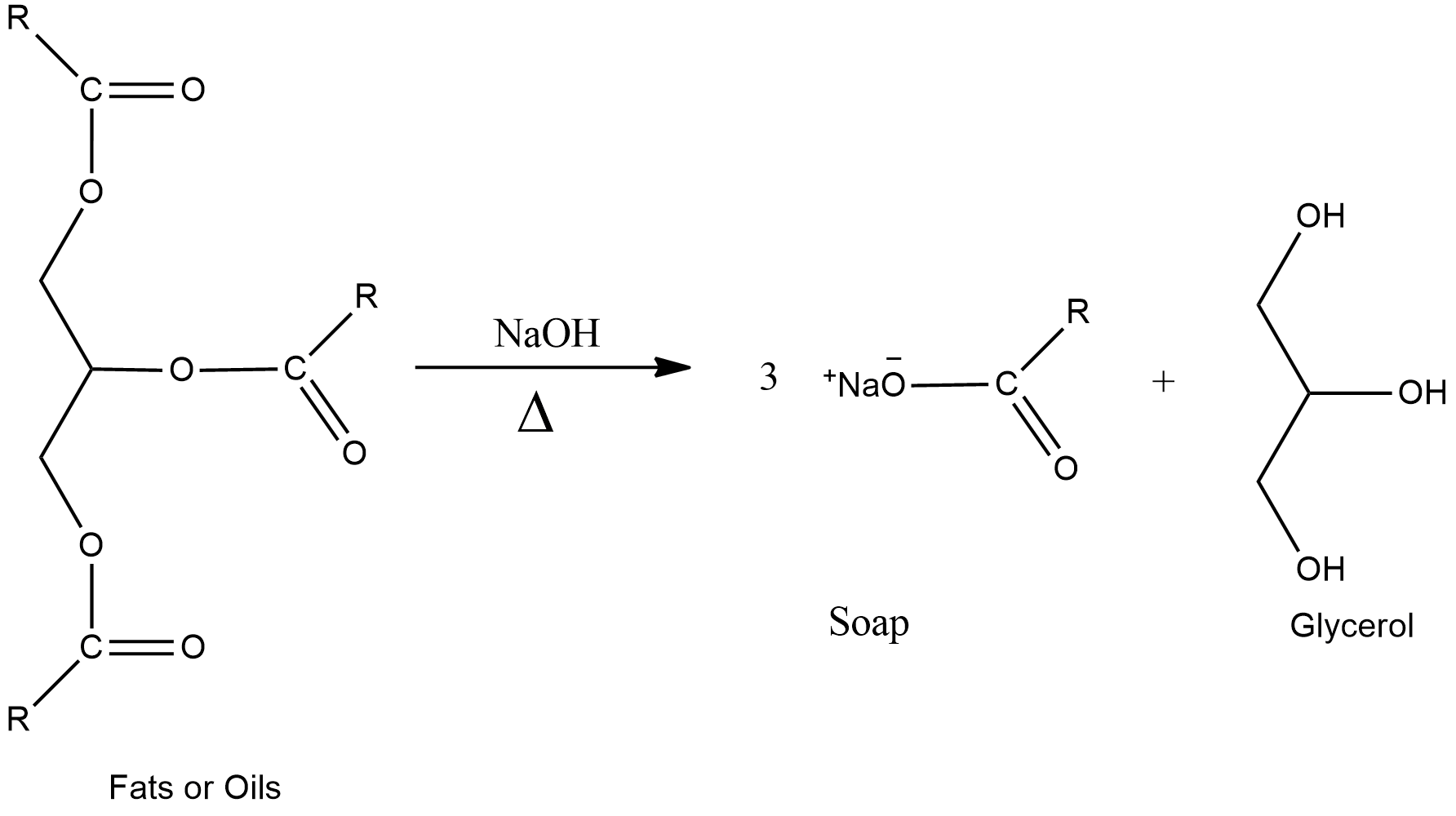
- The fatty acids react with salts and get precipitated out as soap due to the common ion effect and glycerol remains in the salt solution being soluble in salt solution.
- The fatty acids are soluble in the alkaline solution used for hydrolysis and that’s why, salt is added so that sodium salt of fatty acid is formed and it gets precipitated out.
- Potassium soaps are softer than sodium soaps. Potassium soaps are used in shampoo, shaving creams and bathing soaps (liquid or cake). Sodium soaps are toilet soaps used for washing purposes.
- Therefore, sodium chloride is used in the soap industry for soap’s precipitation.
- Therefore, the correct answer is option (C).
Note: Remember soaps are prepared by alkaline hydrolysis of fats or oils. Soaps are salts of fatty acids having a long chain of more than 12 carbon atoms. Soaps are insoluble in salt solution and precipitate out due to common ion effects. Glycerol is soluble in salt solution.
Complete step by step solution:
- The process of manufacturing of soap is known as saponification.
- Soaps are sodium or potassium salts of long chains of fatty acids which contain more than 12 carbon atoms.
- In saponification, fats or oils are first hydrolysed by using sodium hydroxide, NaOH or potassium hydroxide, KOH to form a solution of glycerol and fatty acids.
- Soap is then precipitated from the solution by adding salt like sodium chloride, NaCl, potassium chloride, KCl, etc.

- The fatty acids react with salts and get precipitated out as soap due to the common ion effect and glycerol remains in the salt solution being soluble in salt solution.
- The fatty acids are soluble in the alkaline solution used for hydrolysis and that’s why, salt is added so that sodium salt of fatty acid is formed and it gets precipitated out.
- Potassium soaps are softer than sodium soaps. Potassium soaps are used in shampoo, shaving creams and bathing soaps (liquid or cake). Sodium soaps are toilet soaps used for washing purposes.
- Therefore, sodium chloride is used in the soap industry for soap’s precipitation.
- Therefore, the correct answer is option (C).
Note: Remember soaps are prepared by alkaline hydrolysis of fats or oils. Soaps are salts of fatty acids having a long chain of more than 12 carbon atoms. Soaps are insoluble in salt solution and precipitate out due to common ion effects. Glycerol is soluble in salt solution.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




