
SOLUTION OF POTASSIUM FERROCYANIDE CONTAIN ----- IONS?
(A) $5$
(B) $4$
(C) $10$
(D) $11$
Answer
576k+ views
Hint: Potassium and sodium ferrocyanide, both hydrate and anhydrous salts, has a complex polymeric composition, as most metal cyanides. The polymer consists of octahedral ${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}$-centers crosslinked with $K + $ ions bound to the $CN$ ligands. As the solid is dissolved in water, the ${K^ + } - NC$ linkages break.
Complete step by step answer:
In order to solve this question we first need to know about potassium ferrocyanide , it is a compound with chemical formula ${K_4}[Fe{\left( {CN} \right)_6}]\cdot3{H_2}O$ .It is also known as yellow potash parasite because the salt of potassium ferrocyanide forms lemon yellow mono cyclic crystals.
As the name indicates the compound contain potassium and ferrocyanide where ferrous have an oxidation state of $ + 2$ and cyanide have charge of $ - 1$ so the overall charge of ferrocyanide becomes
$( + 2) + 6( - 1) = - 4$ which require $4$ potassium ions to balance.
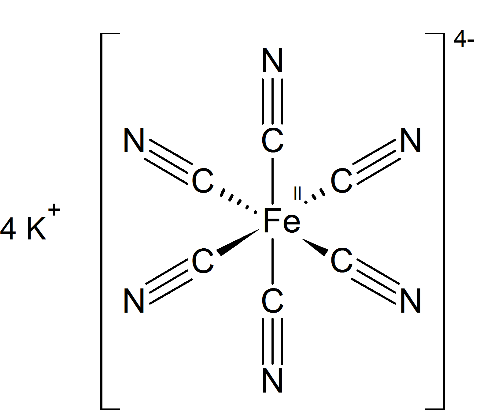
So when considering our question , a solution of potassium ferrocyanide will give $5$ ions
Potassium ferrocyanide ${K_4}\left[ {Fe{{(CN)}_6}} \right]$ will ionize as
${K_4}[Fe{(CN)_6}] \to 4{K^ + } + [Fe{(C{N_6}]^{4 - }}$
So, it will give $5$ ions in the solution
So, the correct answer is Option A.
Additional information:
Potassium ferrocyanide is used in detection of $F{e^{3 + }},C{u^{2 + }},F{e^{2 + }}$ . This is not toxic and does not decompose into cyanide in our body.
In reaction with ferric chloride potassium ferrocyanide gives potassium di iron $(III)$hexacyanoferrate which is dark blue precipitate called Persian blue. Many niche uses of potassium ferrocyanide are found in the industry. Ferrocyanides of potassium and sodium are also used to purify tin and to isolate copper from molybdenum ore. Potassium ferrocyanide is used in wine and citric acid processing.
Note: Do not confuse potassium ferrocyanide with potassium ferricyanide , in potassium ferricyanide ${K_3}[Fe{(CN)_6}]$iron is in $ + 3$ oxidation state and it will give 4 ions on dissociation .
Potassium ferricyanide is diamagnetic whereas potassium ferrocyanide is diamagnetic in nature because crystal field splitting theory of ferrocyanide is greater than that of a ferricyanide
The IUPAC name of potassium ferrocyanide is hexacyanoferrate $(II)$ .
Complete step by step answer:
In order to solve this question we first need to know about potassium ferrocyanide , it is a compound with chemical formula ${K_4}[Fe{\left( {CN} \right)_6}]\cdot3{H_2}O$ .It is also known as yellow potash parasite because the salt of potassium ferrocyanide forms lemon yellow mono cyclic crystals.
As the name indicates the compound contain potassium and ferrocyanide where ferrous have an oxidation state of $ + 2$ and cyanide have charge of $ - 1$ so the overall charge of ferrocyanide becomes
$( + 2) + 6( - 1) = - 4$ which require $4$ potassium ions to balance.

So when considering our question , a solution of potassium ferrocyanide will give $5$ ions
Potassium ferrocyanide ${K_4}\left[ {Fe{{(CN)}_6}} \right]$ will ionize as
${K_4}[Fe{(CN)_6}] \to 4{K^ + } + [Fe{(C{N_6}]^{4 - }}$
So, it will give $5$ ions in the solution
So, the correct answer is Option A.
Additional information:
Potassium ferrocyanide is used in detection of $F{e^{3 + }},C{u^{2 + }},F{e^{2 + }}$ . This is not toxic and does not decompose into cyanide in our body.
In reaction with ferric chloride potassium ferrocyanide gives potassium di iron $(III)$hexacyanoferrate which is dark blue precipitate called Persian blue. Many niche uses of potassium ferrocyanide are found in the industry. Ferrocyanides of potassium and sodium are also used to purify tin and to isolate copper from molybdenum ore. Potassium ferrocyanide is used in wine and citric acid processing.
Note: Do not confuse potassium ferrocyanide with potassium ferricyanide , in potassium ferricyanide ${K_3}[Fe{(CN)_6}]$iron is in $ + 3$ oxidation state and it will give 4 ions on dissociation .
Potassium ferricyanide is diamagnetic whereas potassium ferrocyanide is diamagnetic in nature because crystal field splitting theory of ferrocyanide is greater than that of a ferricyanide
The IUPAC name of potassium ferrocyanide is hexacyanoferrate $(II)$ .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




