
How many species out of the following are aromatic?

Answer
573.6k+ views
Hint: Aromatic compounds are considered as the compound which contains a conjugated planar ring system with delocalized pi-electrons cloud located at alternate double bond and single bond. For a compound to be aromatic it should follow Huckel’s rule which says that the compound should satisfy 4n+2$\pi$ electrons.
Complete answer:
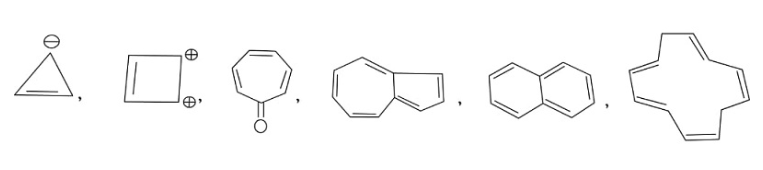
In the above given compound, 1 $\pi$ bond is present which contains 2 electrons. Due to negative charge 2 electrons are present in the same plane. So, the total number of electrons is 4. This compound does not follow the 4n+2$\pi$rule. Therefore, it is antiaromatic.
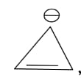
In the above given compound, 1 $\pi$ bond is present which contains 2 electrons. It is an aromatic compound as it is cyclic, and planar. Due to positive charge all the carbon atoms become $s{p^2}$ hybridized and it follows Huckel’s rule.
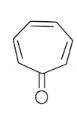
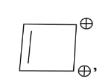
In the above given compound, 3 $\pi$ bond is present which contains 6 electrons. The oxygen atom is not present in the plane, therefore its electrons are not counted. This compound is aromatic as it follows the 4n+2$\pi$rule.
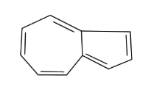
In the above given compound, 3 $\pi$ bond is present which contains 6 electrons. The other cycle is not present in the plane, so its electrons are not counted. This compound is aromatic as it follows the 4n+2$\pi$rule.
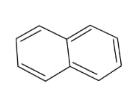
In the above compound, total 5 $\pi$ bonds are present which contains 10 electrons. Therefore, this compound is aromatic as it follows the 4n+2$\pi$rule.
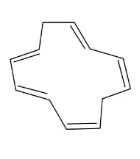
In the above compound, total 5 $\pi$ bonds are present which contains 10 electrons but the $\pi$electrons are not present in conjugation. Therefore, it is an antiaromatic compound.
Note:
In the presence of fused ring structure, the cycle with maximum number of conjugated $\pi$ bonds is considered. The lone pairs which are present within the plane only participate in Huckel’s rule, else they are not considered.
Complete answer:

In the above given compound, 1 $\pi$ bond is present which contains 2 electrons. Due to negative charge 2 electrons are present in the same plane. So, the total number of electrons is 4. This compound does not follow the 4n+2$\pi$rule. Therefore, it is antiaromatic.

In the above given compound, 1 $\pi$ bond is present which contains 2 electrons. It is an aromatic compound as it is cyclic, and planar. Due to positive charge all the carbon atoms become $s{p^2}$ hybridized and it follows Huckel’s rule.

In the above given compound, 3 $\pi$ bond is present which contains 6 electrons. The oxygen atom is not present in the plane, therefore its electrons are not counted. This compound is aromatic as it follows the 4n+2$\pi$rule.

In the above given compound, 3 $\pi$ bond is present which contains 6 electrons. The other cycle is not present in the plane, so its electrons are not counted. This compound is aromatic as it follows the 4n+2$\pi$rule.

In the above compound, total 5 $\pi$ bonds are present which contains 10 electrons. Therefore, this compound is aromatic as it follows the 4n+2$\pi$rule.

In the above compound, total 5 $\pi$ bonds are present which contains 10 electrons but the $\pi$electrons are not present in conjugation. Therefore, it is an antiaromatic compound.
Note:
In the presence of fused ring structure, the cycle with maximum number of conjugated $\pi$ bonds is considered. The lone pairs which are present within the plane only participate in Huckel’s rule, else they are not considered.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




