
State and explain Raoult’s law.
Answer
578.1k+ views
Hint: Raoult’s law explains the change in vapour pressure of the solvent in the solution. Raoult’s law applies to ideal solutions at any concentration while for real solutions law is applicable only to dilute solutions.
Complete step by step answer:
Raoult’s law states that vapour pressure of the solvent in a solution is the product of mole fraction of solvent and vapour pressure of the pure solvent.
The mathematical expression of Raoult’s law is as follows:
${P_{{\text{solution}}}} = {X_{{\text{solvent}}}} \times P_{{\text{solvent}}}^ \circ $
Here,
${P_{{\text{solution}}}}$= vapour pressure of the solution
${X_{{\text{solvent}}}}$ = mole fraction of solvent
$P_{{\text{solvent}}}^ \circ $= vapour pressure of the pure solvent
Raoult’s law is valid for the solution of non-volatile and non-electrolytic solute. The vapour pressure of such a solution is always lower than the vapour pressure of the pure solvent.
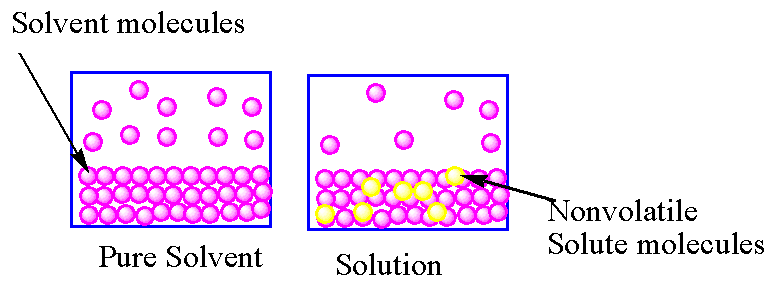
In the case of a pure solvent , the rate of vaporisation and condensation are equal. In case of solution due to the presence of solute particles on the surface of the solvent, the rate of vaporisation is lower than the rate of vaporisation of the pure solvent. So in solution, to achieve equilibrium, the gaseous molecules convert into liquid due to which, the vapour pressure of a solution is lower than the vapour pressure of the pure solvent.
Another factor which causes vapour pressure lowering is the increasing entropy. The solution has higher entropy than pure solvent so the solution has a lower rate of vaporisation than the pure solvent.
Raoult’s law is also applicable for the solution of non-volatile liquid pairs like a solution of benzene and toluene, n-hexane and n-heptane etc.
So, the correct answer is “Option A”.
Note:
Lowering in vapour pressure of a solution is one of the colligative properties explained by Raoult's law. Ideal solutions obey Raoult’s law at all concentration and temperature. Real solutions show deviation to Raoult’s law at higher concentration while dilute solutions obey Raoult's law.
Complete step by step answer:
Raoult’s law states that vapour pressure of the solvent in a solution is the product of mole fraction of solvent and vapour pressure of the pure solvent.
The mathematical expression of Raoult’s law is as follows:
${P_{{\text{solution}}}} = {X_{{\text{solvent}}}} \times P_{{\text{solvent}}}^ \circ $
Here,
${P_{{\text{solution}}}}$= vapour pressure of the solution
${X_{{\text{solvent}}}}$ = mole fraction of solvent
$P_{{\text{solvent}}}^ \circ $= vapour pressure of the pure solvent
Raoult’s law is valid for the solution of non-volatile and non-electrolytic solute. The vapour pressure of such a solution is always lower than the vapour pressure of the pure solvent.

In the case of a pure solvent , the rate of vaporisation and condensation are equal. In case of solution due to the presence of solute particles on the surface of the solvent, the rate of vaporisation is lower than the rate of vaporisation of the pure solvent. So in solution, to achieve equilibrium, the gaseous molecules convert into liquid due to which, the vapour pressure of a solution is lower than the vapour pressure of the pure solvent.
Another factor which causes vapour pressure lowering is the increasing entropy. The solution has higher entropy than pure solvent so the solution has a lower rate of vaporisation than the pure solvent.
Raoult’s law is also applicable for the solution of non-volatile liquid pairs like a solution of benzene and toluene, n-hexane and n-heptane etc.
So, the correct answer is “Option A”.
Note:
Lowering in vapour pressure of a solution is one of the colligative properties explained by Raoult's law. Ideal solutions obey Raoult’s law at all concentration and temperature. Real solutions show deviation to Raoult’s law at higher concentration while dilute solutions obey Raoult's law.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




