
State Moseley’s law. What is its importance?
Answer
592.2k+ views
Hint: Most radiations are distinguished by their corresponding wavelengths and frequencies. An X-ray, also known as X-radiation, is a form of high-energy electromagnetic radiation which is penetrating in nature is dependent upon the atomic number of the surface material. Therefore the energy carried by it is also dependent upon the atomic number.
Complete step by step answer:
The square root of the frequency of the emitted X-ray is proportional to the atomic number. This is known as Moseley’s law. English physicist, Henry Moseley discovered and issued the law.
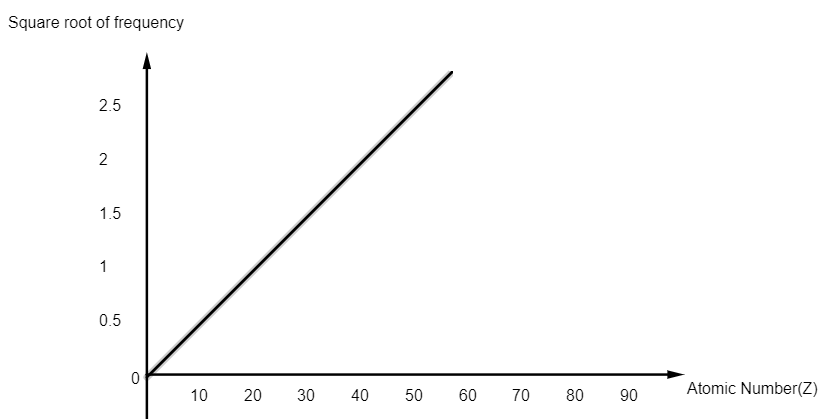
The importance of the law is as follows:
The atomic number was given more importance after the discovery of the law. Atomic number was associated as a measurable physical quantity because of the law.
It led to the discovery of many new elements like Rh, Pr, Tc.
For the rare earth metals, atomic numbers were determined using this law
Additional Information:
When highly energetic electrons are made to strike a metal target, electromagnetic radiations are emitted from the target. A large part of this radiation has a wavelength of order 0.1nm and these radiations are known as X-rays. A device used to produce X-rays is generally called the X-ray tube. In the process of X-ray generation a fraction of kinetic energy appears as the energy of photons varies from collision to collision. In a certain collision, the electron may lose its entire kinetic energy to bring out a photon or it may not create a photon at all. So the energy of the generated photon can lie anywhere between 0 to 1eV.
Note: An easier way for students to memorize the difference between hard and soft X-rays is to associate the term hard with more. Therefore hard X-rays are the one with more energy. And by establishing that they have more energy, by the equation giving relation between wavelength and energy ($E=\dfrac{hc}{\lambda }$) it will be clear that these quantities are inversely proportional. So higher the energy, lower will be the wavelength.
Complete step by step answer:
The square root of the frequency of the emitted X-ray is proportional to the atomic number. This is known as Moseley’s law. English physicist, Henry Moseley discovered and issued the law.

The importance of the law is as follows:
The atomic number was given more importance after the discovery of the law. Atomic number was associated as a measurable physical quantity because of the law.
It led to the discovery of many new elements like Rh, Pr, Tc.
For the rare earth metals, atomic numbers were determined using this law
Additional Information:
When highly energetic electrons are made to strike a metal target, electromagnetic radiations are emitted from the target. A large part of this radiation has a wavelength of order 0.1nm and these radiations are known as X-rays. A device used to produce X-rays is generally called the X-ray tube. In the process of X-ray generation a fraction of kinetic energy appears as the energy of photons varies from collision to collision. In a certain collision, the electron may lose its entire kinetic energy to bring out a photon or it may not create a photon at all. So the energy of the generated photon can lie anywhere between 0 to 1eV.
Note: An easier way for students to memorize the difference between hard and soft X-rays is to associate the term hard with more. Therefore hard X-rays are the one with more energy. And by establishing that they have more energy, by the equation giving relation between wavelength and energy ($E=\dfrac{hc}{\lambda }$) it will be clear that these quantities are inversely proportional. So higher the energy, lower will be the wavelength.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




