
State the electronic configuration for Nitrogen \[\left[ \mathbf{p}=\mathbf{7},\text{ }\mathbf{n}=\mathbf{7} \right]\]
Answer
546.6k+ views
Hint: As we know, atomic number is total number of a proton present in a nucleus of atom as well as atomic mass number is total number of a proton along with neutron. Nitrogen differs from other elements of the group $15$ due to its high electronegative character small size as well as high ionization enthalpy. Nitrogen could form multiple bonds with itself as well as other elements. It forms \[pp\] multiple bonds.
Complete step-by-step answer:The atomic number of nitrogen is $7$ . This is the number of protons in a nuclei of nitrogen atoms. A neutral atom has the same number of electrons as protons. So electron configuration will include $7$ electron placed into appropriate $s$ along with $p$ orbitals in ground state, state of a lowest energy. The full electron configurations for nitrogen is \[1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}\]
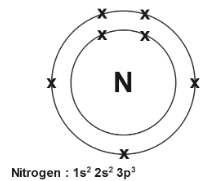
So the nitrogen has an atomic number $7$ as well as mass number \[7+7=14\] , $K$ shell will place $2$ electrons as well as rest will be accommodated into $L$ shell. Therefore configuration is \[2,\text{ }5\] just adding power of configuration also we know that $s$ hold $2$ electron so the electron at $2{{s}^{2}}$ will be at second subshell since second subshell $p$ holds up to $6$ electrons so $2$ electrons from $2{{s}^{2}}$ and $3{{p}^{3}}$ which sums up and gives, $5$ electron in \[p-orbital\]
Therefore, the electronic configuration of Nitrogen is \[2,\text{ }5\].
Note: Note that atomic number of nitrogen is $7$ . This is the number of protons in the nuclei of nitrogen atoms. A neutral atom has the same number of electrons as protons. So the electronic configurations will include $7$ electron placed into appropriate $s$ as well as $p$ orbital in ground state/state of lowest energy.
Complete step-by-step answer:The atomic number of nitrogen is $7$ . This is the number of protons in a nuclei of nitrogen atoms. A neutral atom has the same number of electrons as protons. So electron configuration will include $7$ electron placed into appropriate $s$ along with $p$ orbitals in ground state, state of a lowest energy. The full electron configurations for nitrogen is \[1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}\]

So the nitrogen has an atomic number $7$ as well as mass number \[7+7=14\] , $K$ shell will place $2$ electrons as well as rest will be accommodated into $L$ shell. Therefore configuration is \[2,\text{ }5\] just adding power of configuration also we know that $s$ hold $2$ electron so the electron at $2{{s}^{2}}$ will be at second subshell since second subshell $p$ holds up to $6$ electrons so $2$ electrons from $2{{s}^{2}}$ and $3{{p}^{3}}$ which sums up and gives, $5$ electron in \[p-orbital\]
Therefore, the electronic configuration of Nitrogen is \[2,\text{ }5\].
Note: Note that atomic number of nitrogen is $7$ . This is the number of protons in the nuclei of nitrogen atoms. A neutral atom has the same number of electrons as protons. So the electronic configurations will include $7$ electron placed into appropriate $s$ as well as $p$ orbital in ground state/state of lowest energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




