
State the type of bonding present in hydronium ions.
Answer
558.9k+ views
Hint: In this question, we need to express the type of bonding present in the hydronium ion. For this, we have hydronium ions, so first of all we want to write the electronic configuration and think about different kinds of bonds which suit for the stability of hydronium ions.
Complete step-by-step answer:
First of all we want to write the electronic configuration
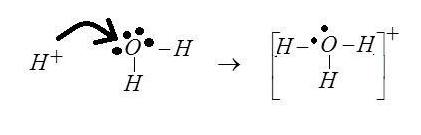
The dative bond is present here is Dative bond (Coordinate covalent bond) .The dative bond is formed when one atom in the bonding provides both bonding electrons where we can see the same situation the oxygen atom is proving both electrons for bonding. Hence the bonding caused here is Dative bonding
DATIVE BONDING: This is a special type of covalent bond in this bonding the shared pair if electron for the formation of bonding is shared by the same atom. The atom which donates the electron is called the donor atom, it will usually be a Lewis base, the other atom which only shares the electron pair is called acceptor, it will usually be a Lewis acid.
Properties:
(1)Usually the molecules which form this type of bonding have melting and boiling points are higher than purely covalent compounds and lower than purely ionic compounds.
(2) These are sparingly soluble in polar solvent like water but readily soluble in non-polar solvents.
(3) The bond is rigid and directional. Thus, coordinate compounds show isomerism.
Note: Student can make in writing the correct electronic configuration of the elements. Also an important concept is that we can image the bonding caused here by drawing their figure, drawing the image of bonding will always make it easy to identify which type of bonding caused in each ion formation.
Complete step-by-step answer:
First of all we want to write the electronic configuration

The dative bond is present here is Dative bond (Coordinate covalent bond) .The dative bond is formed when one atom in the bonding provides both bonding electrons where we can see the same situation the oxygen atom is proving both electrons for bonding. Hence the bonding caused here is Dative bonding
DATIVE BONDING: This is a special type of covalent bond in this bonding the shared pair if electron for the formation of bonding is shared by the same atom. The atom which donates the electron is called the donor atom, it will usually be a Lewis base, the other atom which only shares the electron pair is called acceptor, it will usually be a Lewis acid.
Properties:
(1)Usually the molecules which form this type of bonding have melting and boiling points are higher than purely covalent compounds and lower than purely ionic compounds.
(2) These are sparingly soluble in polar solvent like water but readily soluble in non-polar solvents.
(3) The bond is rigid and directional. Thus, coordinate compounds show isomerism.
Note: Student can make in writing the correct electronic configuration of the elements. Also an important concept is that we can image the bonding caused here by drawing their figure, drawing the image of bonding will always make it easy to identify which type of bonding caused in each ion formation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




