
State the valency of
A) Sulphite
B) Nitrate
C) Hydroxide
Answer
596.1k+ views
Hint: Valency is the number of electrons needed by the element to gain a stable configuration. It can be easily calculated by using the octet rule or by the charge rule. If we want to calculate it by the octet rule, we have to find out the number of electrons in the outermost shell and subtract it by 8 which will give us the number of electrons needed to gain a stable configuration.
Complete step by step answer:
As we know, valency of an element is the measure of its combining power with other atoms when it forms chemical compounds.
We can also write it as the affinity of an atom to give or lose electrons in order to attain a noble gas configuration.
Atoms gain stability by losing, gaining or sharing their electrons present in the outermost shell.
Valency can be calculated by using the octet rule, which states that the elements with 8 electrons in their outermost shell are stable. So if the number of electrons is 5 in their outermost shell, valency is calculated by subtracting 5 from 8 i.e. the element needs to gain 3 electrons to gain stability.
Now we will find out the valency of-
A) Sulphite- The formula of sulphite is$S{{O}_{3}}^{2-}$.
Atomic number of sulphur=16
Electrons distribution of S = 2, 8, 6
As we can see from the above distribution, the number of valence electrons in sulphur is 6. Structure of sulphite is-
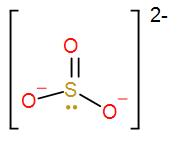
As we can see from the above structure, the 4 electrons are occupied by the 3 oxygen (one double bond two single bonds) i.e. it only needs to gain 2 more electrons to attain stability.
Therefore, valency of sulphite =2
We can also check it from the octet rule, (8-6) electrons needed to = 2
B) Nitrate- The formula of nitrate is $N{{O}_{3}}^{-}$
Atomic number of nitrogen= 7
Distribution of electrons in N= 2, 5
Structure of nitrate-
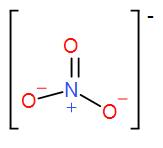
The number of valence electrons in nitrogen= 5
According to octet rule, it needs (8-5) = 3 electrons to gain stability. It already has 4 electrons from the 3 oxygen bonds but it also attained a positive charge so, we will add 1 while calculating the valency which will come out as 1
Therefore, valency of nitrate = 1
C) Hydroxide- The formula of hydroxide is $O{{H}^{-}}$
Atomic number of oxygen= 8
Electronic distribution of O= 2, 6
Therefore, It needs (8-6) =2 more electrons in its outer shell to gain stability. It already has one electron from the hydrogen atom i.e. it needs only 1 more electron to gain stability.
Therefore, valency of hydroxide=1
Note: It is important to remember here that valency is not always equal to the oxidation number of the compound. It is also important to note that there is no positive or negative valency. It is simply the electron affinity. However, it is sometimes denoted as negative if the atom needs to gain electrons to be stable and positive if the atom needs to donate an electron to be stable.
Complete step by step answer:
As we know, valency of an element is the measure of its combining power with other atoms when it forms chemical compounds.
We can also write it as the affinity of an atom to give or lose electrons in order to attain a noble gas configuration.
Atoms gain stability by losing, gaining or sharing their electrons present in the outermost shell.
Valency can be calculated by using the octet rule, which states that the elements with 8 electrons in their outermost shell are stable. So if the number of electrons is 5 in their outermost shell, valency is calculated by subtracting 5 from 8 i.e. the element needs to gain 3 electrons to gain stability.
Now we will find out the valency of-
A) Sulphite- The formula of sulphite is$S{{O}_{3}}^{2-}$.
Atomic number of sulphur=16
Electrons distribution of S = 2, 8, 6
As we can see from the above distribution, the number of valence electrons in sulphur is 6. Structure of sulphite is-

As we can see from the above structure, the 4 electrons are occupied by the 3 oxygen (one double bond two single bonds) i.e. it only needs to gain 2 more electrons to attain stability.
Therefore, valency of sulphite =2
We can also check it from the octet rule, (8-6) electrons needed to = 2
B) Nitrate- The formula of nitrate is $N{{O}_{3}}^{-}$
Atomic number of nitrogen= 7
Distribution of electrons in N= 2, 5
Structure of nitrate-

The number of valence electrons in nitrogen= 5
According to octet rule, it needs (8-5) = 3 electrons to gain stability. It already has 4 electrons from the 3 oxygen bonds but it also attained a positive charge so, we will add 1 while calculating the valency which will come out as 1
Therefore, valency of nitrate = 1
C) Hydroxide- The formula of hydroxide is $O{{H}^{-}}$
Atomic number of oxygen= 8
Electronic distribution of O= 2, 6
Therefore, It needs (8-6) =2 more electrons in its outer shell to gain stability. It already has one electron from the hydrogen atom i.e. it needs only 1 more electron to gain stability.
Therefore, valency of hydroxide=1
Note: It is important to remember here that valency is not always equal to the oxidation number of the compound. It is also important to note that there is no positive or negative valency. It is simply the electron affinity. However, it is sometimes denoted as negative if the atom needs to gain electrons to be stable and positive if the atom needs to donate an electron to be stable.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




