
State True or False
The graph between PV vs P at constant temperature is linear parallel to the pressure axis.
A. True
B. False
Answer
599.1k+ views
Hint: “When temperature is constant, then the product of pressure and volume is constant. This relationship is known as Boyle's law or Mariotte's law”. At constant temperature the process is called as isothermal.
Complete step by step answer:
- We know that the Ideal gas equation is PV= nRT where P = Pressure V = Volume n = Number of moles of gas R = Universal, gas constant. T = Temperature of the gas
Subsequently if temperature is kept constant the RHS of the equation (nRT) is also constant.
Therefore PV= Constant
If we are going to draw a graph, PV against P, it will be a straight line parallel to the P axis.
So, the given statement is true.
So, the correct option is A.
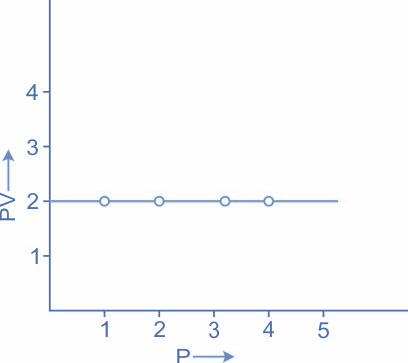
The above graph explains the same concept, a graph, PV against P it will be a straight line parallel to the P axis.
Note: Don’t be confused with the words isothermal and isobaric process. Both are different.
Isothermal process: Isothermal process is a thermodynamic process in which the temperature is constant
Isobaric process: Isobaric process is a thermodynamic process in which the pressure is constant.
If “PV = constant”, then it is called as Boyle’s law, here temperature is constant.
Complete step by step answer:
- We know that the Ideal gas equation is PV= nRT where P = Pressure V = Volume n = Number of moles of gas R = Universal, gas constant. T = Temperature of the gas
Subsequently if temperature is kept constant the RHS of the equation (nRT) is also constant.
Therefore PV= Constant
If we are going to draw a graph, PV against P, it will be a straight line parallel to the P axis.
So, the given statement is true.
So, the correct option is A.

The above graph explains the same concept, a graph, PV against P it will be a straight line parallel to the P axis.
Note: Don’t be confused with the words isothermal and isobaric process. Both are different.
Isothermal process: Isothermal process is a thermodynamic process in which the temperature is constant
Isobaric process: Isobaric process is a thermodynamic process in which the pressure is constant.
If “PV = constant”, then it is called as Boyle’s law, here temperature is constant.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




