
Statement $1$ : Bromobenzene upon reaction with $B{r_2}/Fe$ gives $1,4 - $ dibromobenzene as the major product.
Statement $2$ : In bromobenzene, the inductive effect of the bromo group is more dominant than the mesomeric effect in directing the incoming electrophile.
A.Statement $1$ is true, statement $2$ is true, the statement $2$ is the correct explanation for statement $1$ .
B.Statement $1$ is true, statement $2$ is true, the statement $2$ is not a correct explanation for statement $1$.
C.Statement $1$ is true and statement $2$ is false.
D.Statement $1$ is false and statement $2$ is true.
Answer
570.9k+ views
Hint: Bromobenzene is an unsaturated compound which can undergo addition reactions easily. In this bromine is an ortho and para directing group. there is only one type of effect which takes place in this unsaturated compound.
Complete answer:
A.Statement $1$ : When bromobenzene reacts with $B{r_2}/Fe$ it gives $1,4 - $ dibromobenzene as the major product.
The reaction is as follows:
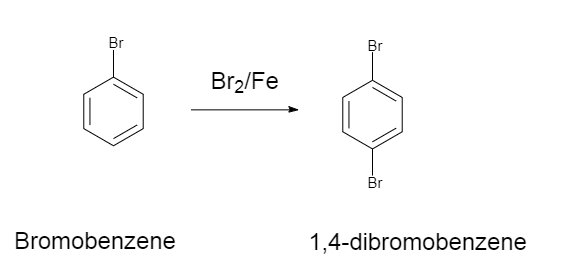
Since bromine is ortho and para directing, there is a mesomeric effect.
$1,2 - $dibromobenzene is not possible because it will cause bulkiness and also there will be repulsion which will cause instability.
Whereas $1,4 - $dibromobenzene are symmetric to each other and will cause very less repulsion.
That is why we get $1,4 - $ dibromobenzene as the major product.
Therefore, the statement $1$ is true.
B.Statement $2$ : in bromobenzene, the inductive effect of the bromo group is more dominant than the mesomeric effect in directing the incoming electrophile.
The statement $2$ is false because in bromobenzene it is due to mesomeric effect which directs incoming electrophile to attack on ortho and para position.
Mesomeric effect depends on the functional groups or substituents present in a chemical compound.
Inductive effect is the difference in the electronegativity between the two atoms in the bond.
So the mesomeric effect is more dominant than the inductive effect in the case of bromobenzene . Therefore the correct answer is option (C) Statement $1$ is true and statement $2$ is false.
Note: Inductive effect takes place in saturated compounds and mesomeric effect takes place in unsaturated compounds that have conjugated systems. Inductive effect is the permanent state of polarization and mesomeric effect is caused due to delocalization of electrons.
Complete answer:
A.Statement $1$ : When bromobenzene reacts with $B{r_2}/Fe$ it gives $1,4 - $ dibromobenzene as the major product.
The reaction is as follows:

Since bromine is ortho and para directing, there is a mesomeric effect.
$1,2 - $dibromobenzene is not possible because it will cause bulkiness and also there will be repulsion which will cause instability.
Whereas $1,4 - $dibromobenzene are symmetric to each other and will cause very less repulsion.
That is why we get $1,4 - $ dibromobenzene as the major product.
Therefore, the statement $1$ is true.
B.Statement $2$ : in bromobenzene, the inductive effect of the bromo group is more dominant than the mesomeric effect in directing the incoming electrophile.
The statement $2$ is false because in bromobenzene it is due to mesomeric effect which directs incoming electrophile to attack on ortho and para position.
Mesomeric effect depends on the functional groups or substituents present in a chemical compound.
Inductive effect is the difference in the electronegativity between the two atoms in the bond.
So the mesomeric effect is more dominant than the inductive effect in the case of bromobenzene . Therefore the correct answer is option (C) Statement $1$ is true and statement $2$ is false.
Note: Inductive effect takes place in saturated compounds and mesomeric effect takes place in unsaturated compounds that have conjugated systems. Inductive effect is the permanent state of polarization and mesomeric effect is caused due to delocalization of electrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




