
Statement – I: Aniline on reaction with \[NaN{O_2}/HCl\] at \[0^{0}C\] followed by coupling with β-naphthol gives a dark blue coloured precipitate.
Statement – II: The colour of the compound formed in the reaction of aniline with \[NaN{O_2}/HCl\] at \[0^{0}C\] followed by coupling with β-naphthol.
(A) Statement - 1 is True; Statement - II is True ; Statement - II is a correct explanation for Statement - 1
(B) Statement - I is True; Statement - II is True; Statement - II is Not a correct explanation for Statement - I
(C) Statement - I is True; Statement - II is False
(D) Statement - I is False; Statement - II is True.
Answer
564.9k+ views
Hint:Conversion of the primary aromatic amines to their diazonium salt is known as diazotization. Diazonium salts are essential synthetic intermediates which undergo coupling reactions in order to form azo dyes or can undergo electrophilic substitution reactions in order to introduce functional groups.
Complete step-by-step answer: Let us discuss the given statements one by one.
Statement I: Aniline reacts with \[NaN{O_2}/HCl\] at 273 K to form a diazonium salt as shown in the reaction below. Benzene diazonium chloride exists as a colourless solid which is soluble in polar solvents like water.
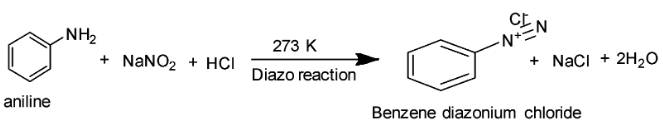
Benzene diazonium chloride is then coupled with β-naphthol to yield a dark red or orange coloured dye as shown in the reaction below:

Thus, the statement I is false as the colour of the precipitate formed is orange red not blue.
Statement II: As we have already seen in the aforementioned reactions that the colour of the compound (dye) formed in the reaction of aniline with \[NaN{O_2}/HCl\] at \[0^{0}C\] was due to the successive coupling of diazonium salt with β-naphthol. Hence, statement II is true.
As a result, Statement - I is False; Statement - II is True.
Hence, the correct answer is Option (D).
Note:Aromatic azo compounds are usually of bright colour owing to the occurrence of extended conjugation. Many of these compounds are used as dyes including methyl red or pigment red 170. Azo printing also employs the same reaction. Azo coupling is even used in the production of sulfa drugs and prontosil.
Complete step-by-step answer: Let us discuss the given statements one by one.
Statement I: Aniline reacts with \[NaN{O_2}/HCl\] at 273 K to form a diazonium salt as shown in the reaction below. Benzene diazonium chloride exists as a colourless solid which is soluble in polar solvents like water.

Benzene diazonium chloride is then coupled with β-naphthol to yield a dark red or orange coloured dye as shown in the reaction below:

Thus, the statement I is false as the colour of the precipitate formed is orange red not blue.
Statement II: As we have already seen in the aforementioned reactions that the colour of the compound (dye) formed in the reaction of aniline with \[NaN{O_2}/HCl\] at \[0^{0}C\] was due to the successive coupling of diazonium salt with β-naphthol. Hence, statement II is true.
As a result, Statement - I is False; Statement - II is True.
Hence, the correct answer is Option (D).
Note:Aromatic azo compounds are usually of bright colour owing to the occurrence of extended conjugation. Many of these compounds are used as dyes including methyl red or pigment red 170. Azo printing also employs the same reaction. Azo coupling is even used in the production of sulfa drugs and prontosil.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




