
Statement- I: Chloral hydrate is stable.
Statement- II: It is stable due to its high molecular weight.
Read the above statements and choose the correct option regarding it.
A.Both the statements are true and statement II is correct explanation of statement I
B.Both the statements are true and statement II is not correct explanation of statement I
C.Statement I is true but statement II is false
D.statement I is false but Statement II is true
Answer
580.8k+ views
Hint: Chloral hydrate consists of water molecules interacting with the chloral molecule.
Molecular weight is the sum of atomic weights of all the atoms present in the molecule.
Complete step by step answer:
Hydrogen bonding is a special type of dipole-dipole attraction between molecules, not a covalent bond to a hydrogen atom. It results from the attractive force between a hydrogen atom covalently bonded to a very electronegative atom such as a \[N,\text{ }O,\text{ }F\] atom and another very electronegative atom. As we all know attractive forces release energy which in turn results in stability of the molecule.
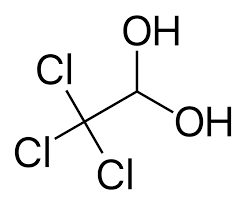
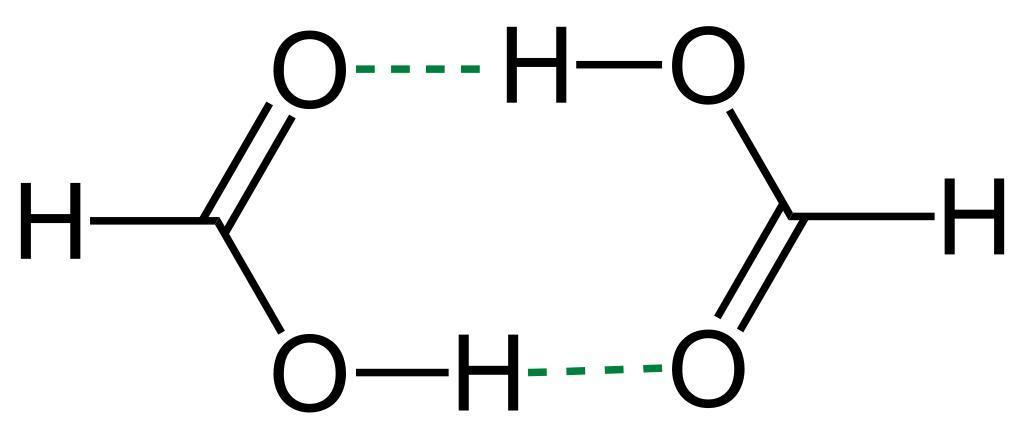
This diagram gives a rough idea about how hydrogen bonding takes place between two polar molecules. In the chloral hydrate, the oxygen of hydroxide interacts with hydrogen of water molecule, and hydrogen of hydroxide interacts with oxygen of water molecule.
The molecular weight does not justify the stability of this molecule, so the statement-II is not correct.
So, the correct answer is Option C .
Additional Information:
Molecules of water are also attracted to other polar molecules and to ions. A polar or charged substance that interacts with and dissolves in water is said to be hydrophilic in nature: hydro means "water," and philic means "loving." In contrast, nonpolar molecules like oils, fats and lipids do not interact well with water. They separate from it rather than dissolve in it and are called hydrophobic in nature: phobic means "fearing." The hydrophobic nature of oils could be easily demonstrated by simply adding oil with water, after sometime the two layers get separated and can be seen distinctively.
Note:
The DNA base pairs, namely ATGC, are bonded with each other by H-bonding, in the double helical structure.
The hydrogen bond can only be established with fluorine, oxygen and nitrogen atoms present in a molecule.
Molecular weight is the sum of atomic weights of all the atoms present in the molecule.
Complete step by step answer:
Hydrogen bonding is a special type of dipole-dipole attraction between molecules, not a covalent bond to a hydrogen atom. It results from the attractive force between a hydrogen atom covalently bonded to a very electronegative atom such as a \[N,\text{ }O,\text{ }F\] atom and another very electronegative atom. As we all know attractive forces release energy which in turn results in stability of the molecule.


This diagram gives a rough idea about how hydrogen bonding takes place between two polar molecules. In the chloral hydrate, the oxygen of hydroxide interacts with hydrogen of water molecule, and hydrogen of hydroxide interacts with oxygen of water molecule.
The molecular weight does not justify the stability of this molecule, so the statement-II is not correct.
So, the correct answer is Option C .
Additional Information:
Molecules of water are also attracted to other polar molecules and to ions. A polar or charged substance that interacts with and dissolves in water is said to be hydrophilic in nature: hydro means "water," and philic means "loving." In contrast, nonpolar molecules like oils, fats and lipids do not interact well with water. They separate from it rather than dissolve in it and are called hydrophobic in nature: phobic means "fearing." The hydrophobic nature of oils could be easily demonstrated by simply adding oil with water, after sometime the two layers get separated and can be seen distinctively.
Note:
The DNA base pairs, namely ATGC, are bonded with each other by H-bonding, in the double helical structure.
The hydrogen bond can only be established with fluorine, oxygen and nitrogen atoms present in a molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




