
How many stereoisomers are $1,2-dichlorocyclopentane$ ?
Answer
535.5k+ views
Hint: To determine the number of stereoisomers of a compound, first determine the number of chiral centers in that molecule. Then, calculate the maximum number of possible stereoisomers of that compound. After this, eliminate if any meso compound is formed. There is a formula to calculate the number of stereoisomers.
Number of stereoisomers $={{2}^{n}}-meso\left( structure \right)$
Where n = number of chiral centers.
Complete step by step solution:
In case of $1,2-dichlorocyclopentane$ , we can easily see that there are two chiral centers in the molecule. Now, we can calculate the maximum number of stereoisomers of the compound which is equal to ${{2}^{2}}=4$
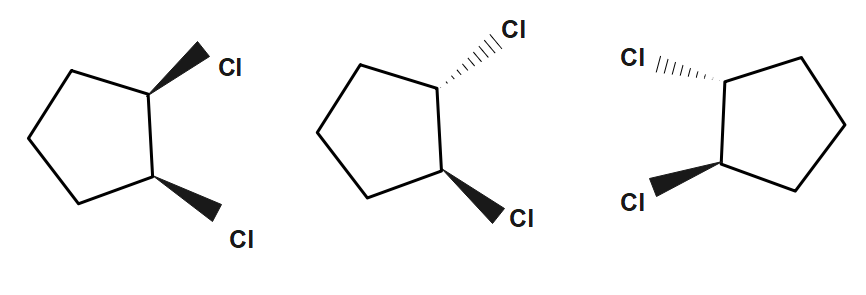
In the cis isomer the two groups point in the same direction relative to the plane of the ring. While in the Trans isomer the two groups point in the opposite direction. (Plane of symmetry present in the two compounds passing through the carbon four and bisecting the carbon one and carbon two bond). Hence the total number of stereoisomers of $1,2-dichlorocyclopentane$is $4-1=3$
So, $3$ is the required answer.
Note: Meso compounds are optically inactive due to the presence of a plane of symmetry in them. Despite containing two or more chiral centers, these compounds are not optically active.
If there is any plane of symmetry existing in a molecule then only meso compounds are going to form. There is no relation between chiral centers and mesostructures. Plane of symmetry means an imaginary plane should divide any molecule into two identical halves then the molecule contains a plane of symmetry
Number of stereoisomers $={{2}^{n}}-meso\left( structure \right)$
Where n = number of chiral centers.
Complete step by step solution:
In case of $1,2-dichlorocyclopentane$ , we can easily see that there are two chiral centers in the molecule. Now, we can calculate the maximum number of stereoisomers of the compound which is equal to ${{2}^{2}}=4$

In the cis isomer the two groups point in the same direction relative to the plane of the ring. While in the Trans isomer the two groups point in the opposite direction. (Plane of symmetry present in the two compounds passing through the carbon four and bisecting the carbon one and carbon two bond). Hence the total number of stereoisomers of $1,2-dichlorocyclopentane$is $4-1=3$
So, $3$ is the required answer.
Note: Meso compounds are optically inactive due to the presence of a plane of symmetry in them. Despite containing two or more chiral centers, these compounds are not optically active.
If there is any plane of symmetry existing in a molecule then only meso compounds are going to form. There is no relation between chiral centers and mesostructures. Plane of symmetry means an imaginary plane should divide any molecule into two identical halves then the molecule contains a plane of symmetry
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




