
Stereoisomers have different:
$ (i) $ molecular mass
$ (ii) $ molecular formula
$ (iii) $ structural formula
$ (iv) $ configuration
Answer
501.3k+ views
Hint: Isomers are those compounds which have the same molecular formula but they have different structural formulas. Stereoisomers are one of the types of isomers which have the same molecular formula but they have different spatial arrangement of atoms . They do not differ by functional groups but they differ by their arrangement of atoms or molecules.
Complete answer:
Isomers refer to that class of organic molecules which have the same molecular formula but they differ with each other in terms of the structural formula. Basically isomers are classify into two types:
$ (i) $ Constitutional or Structural Isomers: These isomers generally differ in functional group, carbon chain, position of the functional group and tautomer.
$ (ii) $ Stereoisomers: These isomers have the same molecular formula but they differ from each other in terms of arrangement of atoms. They can be further classified as,
$ (a.) $ Conformational Isomers
$ (b.) $ Configurational Isomers
$ (c.) $ Diastereomers
$ (d.) $ Enantiomers
All these categories of the stereoisomers are based on the different configuration of atoms present in the molecule. We can also differentiate them by looking at their images. Thus isomers are optically active.
Example of stereoisomer:
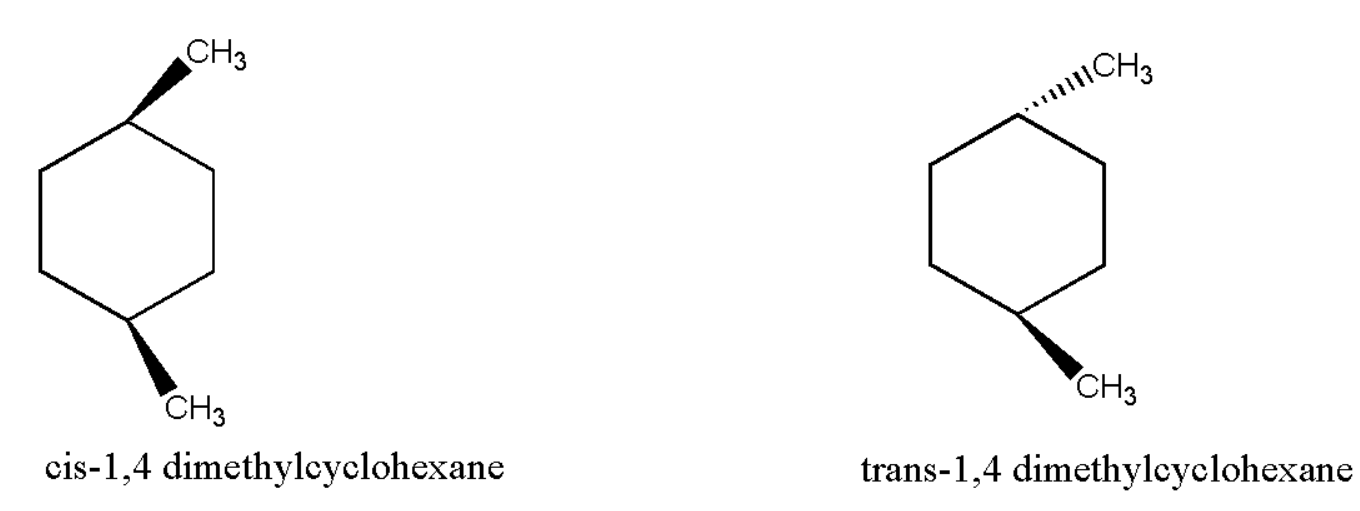
Therefore we can say that in one molecule both the methyl atoms are pointed into the plane while in the other one methyl is pointed into the plane while the other is pointed outward. Hence we can observe that the suitable option for stereoisomers is $ (iv) $ configuration.
Note:
There are other stereoisomers which are optically active while some are not. Basically the configuration in each stereoisomer is different from each other. We can find the structural isomers by looking at their two dimensional image but in the case of stereoisomers we look into the three dimensional image of the molecule.
Complete answer:
Isomers refer to that class of organic molecules which have the same molecular formula but they differ with each other in terms of the structural formula. Basically isomers are classify into two types:
$ (i) $ Constitutional or Structural Isomers: These isomers generally differ in functional group, carbon chain, position of the functional group and tautomer.
$ (ii) $ Stereoisomers: These isomers have the same molecular formula but they differ from each other in terms of arrangement of atoms. They can be further classified as,
$ (a.) $ Conformational Isomers
$ (b.) $ Configurational Isomers
$ (c.) $ Diastereomers
$ (d.) $ Enantiomers
All these categories of the stereoisomers are based on the different configuration of atoms present in the molecule. We can also differentiate them by looking at their images. Thus isomers are optically active.
Example of stereoisomer:

Therefore we can say that in one molecule both the methyl atoms are pointed into the plane while in the other one methyl is pointed into the plane while the other is pointed outward. Hence we can observe that the suitable option for stereoisomers is $ (iv) $ configuration.
Note:
There are other stereoisomers which are optically active while some are not. Basically the configuration in each stereoisomer is different from each other. We can find the structural isomers by looking at their two dimensional image but in the case of stereoisomers we look into the three dimensional image of the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




