
What is steric strain in organic chemistry?
Answer
516.6k+ views
Hint: In chemistry, strain occurs as a molecule's chemical structure is stressed, increasing its internal energy in contrast to a strain-free reference compound. All of the energy contained within a molecule makes up the molecule's intrinsic energy. An unstrained molecule does not have any internal energy, while a stressed molecule does. This additional internal energy, also known as strain energy, can be compared to a compressed spring. The bonds within a molecule will hold a molecule in an energetically favorable conformation, similar to how a compressed spring would be kept in place to avoid the release of its potential energy.
Complete answer:
Nonbonding interactions that affect the shape (conformation) and reactivity of ions and molecules are known as steric effects. Electronic effects, which determine the structure and reactivity of molecules, are complemented by steric effects. The way opposites attract and like charges repel results in coordinated groupings of molecules stabilised by steric repulsive forces between interacting electron clouds. As a result of steric effects, steric hindrance occurs. The slowing of chemical reactions caused by steric bulk is known as steric hindrance. It normally manifests itself in intermolecular reactions, while steric effects are usually discussed in terms of intramolecular interactions.
Steric hindrance is often used to manipulate selectivity, such as by slowing undesirable side effects.
Torsional bond angles may also be affected by steric hindrance between adjacent groups.
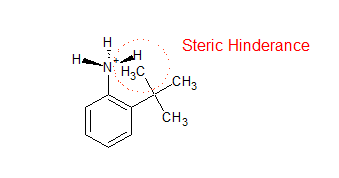
The importance of steric effects in chemistry, biochemistry, and pharmacology cannot be overstated. Steric effects are almost common in organic chemistry, and they influence the rates and activation energies of most chemical reactions to differing degrees. Steric properties are often exploited in biochemistry in naturally occurring molecules such as enzymes, where the catalytic site can be hidden within a large protein structure. Steric effects in pharmacology decide how and at what extent a drug interacts with its target biomolecules.
Note:
As atoms are pushed to get closer than their Van der Waals radii afford, Van der Waals strain, also known as steric strain, occurs. Van der Waals strain is described as a type of strain in which the interacting atoms are separated by at least four bonds. The amount of steric strain in identical molecules is determined by the size of the interacting groups; bulky tert-butyl groups take up much more space than methyl groups, and thus have more steric interactions.
Complete answer:
Nonbonding interactions that affect the shape (conformation) and reactivity of ions and molecules are known as steric effects. Electronic effects, which determine the structure and reactivity of molecules, are complemented by steric effects. The way opposites attract and like charges repel results in coordinated groupings of molecules stabilised by steric repulsive forces between interacting electron clouds. As a result of steric effects, steric hindrance occurs. The slowing of chemical reactions caused by steric bulk is known as steric hindrance. It normally manifests itself in intermolecular reactions, while steric effects are usually discussed in terms of intramolecular interactions.
Steric hindrance is often used to manipulate selectivity, such as by slowing undesirable side effects.
Torsional bond angles may also be affected by steric hindrance between adjacent groups.

The importance of steric effects in chemistry, biochemistry, and pharmacology cannot be overstated. Steric effects are almost common in organic chemistry, and they influence the rates and activation energies of most chemical reactions to differing degrees. Steric properties are often exploited in biochemistry in naturally occurring molecules such as enzymes, where the catalytic site can be hidden within a large protein structure. Steric effects in pharmacology decide how and at what extent a drug interacts with its target biomolecules.
Note:
As atoms are pushed to get closer than their Van der Waals radii afford, Van der Waals strain, also known as steric strain, occurs. Van der Waals strain is described as a type of strain in which the interacting atoms are separated by at least four bonds. The amount of steric strain in identical molecules is determined by the size of the interacting groups; bulky tert-butyl groups take up much more space than methyl groups, and thus have more steric interactions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




