
What is the strongest base?
A.Pyrrole
B.Aniline
C.Pyridine
D.

Answer
584.1k+ views
Hint:We have to know that the basicity of heterocyclic amine differs over a wide range and reflects on both the hybridization of nitrogen orbital consisting of the lone pair of electrons and affects the delocalization.
Complete step by step answer:
We have to know that the order of basicity is,
${\text{s}}{{\text{p}}^{\text{3}}}\left( {\text{N}} \right){\text{ > s}}{{\text{p}}^{\text{2}}}\left( {\text{N}} \right){\text{ > sp}}\left( {\text{N}} \right)$
Pyrrole is a weak base. The lone pair of electrons of the nitrogen atoms interacts with four electrons of the two ${\text{C = C}}$ bonds to produce an aromatic six ${\text{\pi }}$ electron system same to that of benzene.
Therefore, the option (A) is incorrect.
Pyridine is a substantially weaker base than alkylamines like piperidine. The electron pair of pyridine takes place \[{\text{s}}{{\text{p}}^{\text{2}}}\]-hybridized orbital and lies closer to the nucleus than the electron pair in the \[{\text{s}}{{\text{p}}^{\text{3}}}\]-hybridized orbital of alkylamines. Pyridine is a weaker base.
Therefore, the option (B) is incorrect.
In aniline, the lone pair of electrons present in nitrogen could interact with the pi-system of the aromatic ring and this makes them less unused for donation.
Therefore, the option (C) is incorrect.
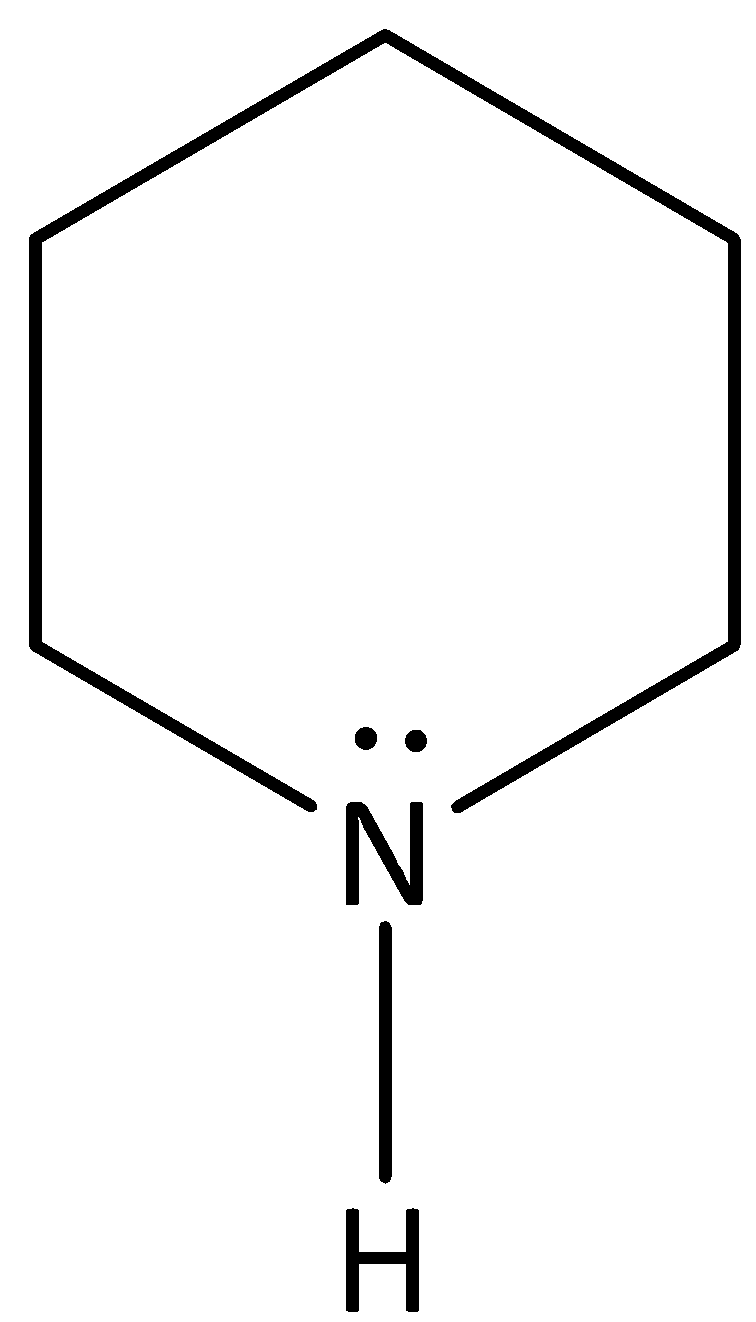
Structure (D) is piperidine.
Aliphatic amines are more bases than aromatic amines due to non-delocalized of lone pairs of nitrogen. The lone pair is piperidine \[{\text{s}}{{\text{p}}^{\text{3}}}\]-hybridized orbital and there is no resonance (no pi system). Piperidine is more basic than others.
Therefore, the option (D) is correct.
The basicity of the given molecule is listed below as,
The ${\text{p}}{{\text{K}}_{\text{a}}}$ of piperidine is 11.2.
The ${\text{p}}{{\text{K}}_{\text{a}}}$ of pyridine is 5.2.
The ${\text{p}}{{\text{K}}_{\text{a}}}$ of aniline is 5.2.
The ${\text{p}}{{\text{K}}_{\text{a}}}$ of pyrrole is 4.
Therefore, the option D is correct.
Note:
We can use pyridine as a flavoring agent. The pyridine ring is a segment of two B vitamins namely niacin and pyridoxine. Niacin is otherwise called as nicotinic acid and is found in most organisms. Heterocyclic amines are carcinogens and are also known as HCA.
Complete step by step answer:
We have to know that the order of basicity is,
${\text{s}}{{\text{p}}^{\text{3}}}\left( {\text{N}} \right){\text{ > s}}{{\text{p}}^{\text{2}}}\left( {\text{N}} \right){\text{ > sp}}\left( {\text{N}} \right)$
Pyrrole is a weak base. The lone pair of electrons of the nitrogen atoms interacts with four electrons of the two ${\text{C = C}}$ bonds to produce an aromatic six ${\text{\pi }}$ electron system same to that of benzene.
Therefore, the option (A) is incorrect.
Pyridine is a substantially weaker base than alkylamines like piperidine. The electron pair of pyridine takes place \[{\text{s}}{{\text{p}}^{\text{2}}}\]-hybridized orbital and lies closer to the nucleus than the electron pair in the \[{\text{s}}{{\text{p}}^{\text{3}}}\]-hybridized orbital of alkylamines. Pyridine is a weaker base.
Therefore, the option (B) is incorrect.
In aniline, the lone pair of electrons present in nitrogen could interact with the pi-system of the aromatic ring and this makes them less unused for donation.
Therefore, the option (C) is incorrect.
Structure (D) is piperidine.
Aliphatic amines are more bases than aromatic amines due to non-delocalized of lone pairs of nitrogen. The lone pair is piperidine \[{\text{s}}{{\text{p}}^{\text{3}}}\]-hybridized orbital and there is no resonance (no pi system). Piperidine is more basic than others.
Therefore, the option (D) is correct.
The basicity of the given molecule is listed below as,
The ${\text{p}}{{\text{K}}_{\text{a}}}$ of piperidine is 11.2.
The ${\text{p}}{{\text{K}}_{\text{a}}}$ of pyridine is 5.2.
The ${\text{p}}{{\text{K}}_{\text{a}}}$ of aniline is 5.2.
The ${\text{p}}{{\text{K}}_{\text{a}}}$ of pyrrole is 4.
Therefore, the option D is correct.
Note:
We can use pyridine as a flavoring agent. The pyridine ring is a segment of two B vitamins namely niacin and pyridoxine. Niacin is otherwise called as nicotinic acid and is found in most organisms. Heterocyclic amines are carcinogens and are also known as HCA.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




