
What is the structural formula of Aspirin?
A)
B)
C)
D)




Answer
593.4k+ views
Hint: Salicylic acid serves as the starting material to prepare aspirin by acetylation. Acetylation is the process of addition of acetyl group (\[C{{H}_{3}}CO\]). Basic functional groups present in aspirin are ester and carboxylic acid.
Complete answer:
Salicylic acid is the common name of 2-hydroxybenzoic acid. It is acetylated using acetic anhydride \[{{(C{{H}_{3}}CO)}_{2}}O\] to give 2-acetoxybenzoic acid which is commonly known as aspirin.
Let us examine all the option given above one by one:
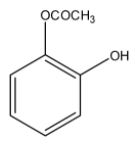
> Option (A): The compound given is 2- acetoxy phenol. It does have an ester group but instead of an acid functional group hydroxyl group is present. So, (A) is not correct.
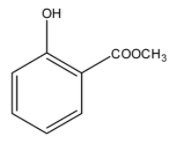
> Option (B): Ester group is present in the compound but acid group is missing. It cannot be correct since it is a methyl ester of salicylic acid.
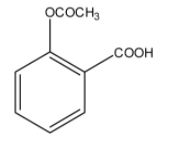
> Option (C): The given compound has both ester and acid functional groups. It is an acetyl derivative of salicylic acid or 2-hydroxybenzoic acid. It is, therefore, the correct structural formula of aspirin.
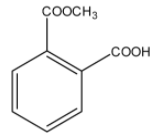
> Option (D): Though both the ester and acid functional groups are present in the compound, it is a mono-methyl ester of phthalic acid. So, it is not the correct answer.
Hence, the correct option is (C).
Additional Information Aspirin acts as a pain reliever. It reduces pain by inhibiting the synthesis of prostaglandins, chemicals that cause tissue inflammation and pain. It is a type of non-narcotic analgesics. It has anti-blood clotting properties and, therefore, is widely used to prevent heart attacks.
Synthesis of Aspirin:
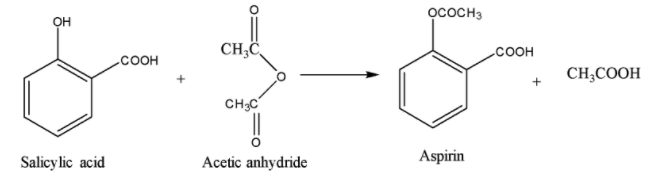
Note: Do not mistake the structure given in option (D) as the answer. It might look similar to the structure of aspirin. Carefully check the connectivity of the carbon with the group present at position-2 with respect to the acid group on the benzene ring.
Complete answer:
Salicylic acid is the common name of 2-hydroxybenzoic acid. It is acetylated using acetic anhydride \[{{(C{{H}_{3}}CO)}_{2}}O\] to give 2-acetoxybenzoic acid which is commonly known as aspirin.
Let us examine all the option given above one by one:
> Option (A): The compound given is 2- acetoxy phenol. It does have an ester group but instead of an acid functional group hydroxyl group is present. So, (A) is not correct.
> Option (B): Ester group is present in the compound but acid group is missing. It cannot be correct since it is a methyl ester of salicylic acid.
> Option (C): The given compound has both ester and acid functional groups. It is an acetyl derivative of salicylic acid or 2-hydroxybenzoic acid. It is, therefore, the correct structural formula of aspirin.
> Option (D): Though both the ester and acid functional groups are present in the compound, it is a mono-methyl ester of phthalic acid. So, it is not the correct answer.
Hence, the correct option is (C).
Additional Information Aspirin acts as a pain reliever. It reduces pain by inhibiting the synthesis of prostaglandins, chemicals that cause tissue inflammation and pain. It is a type of non-narcotic analgesics. It has anti-blood clotting properties and, therefore, is widely used to prevent heart attacks.
Synthesis of Aspirin:

Note: Do not mistake the structure given in option (D) as the answer. It might look similar to the structure of aspirin. Carefully check the connectivity of the carbon with the group present at position-2 with respect to the acid group on the benzene ring.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




