
What is the structural formula of cyclobutane?
Answer
528.9k+ views
Hint: From the name of the compound, we can infer that
- It has a cyclic structure
- It has four carbon atoms
- It does not have any multiple bonds
Complete answer:
We know that cyclobutane is a type of cycloalkane. A cycloalkane is a monocyclic saturated hydrocarbon. It does not contain any atoms or elements except hydrogen and carbon.
Since we know that it has a saturated monocyclic structure and has four carbon atoms, its Lewis structure will be as follows
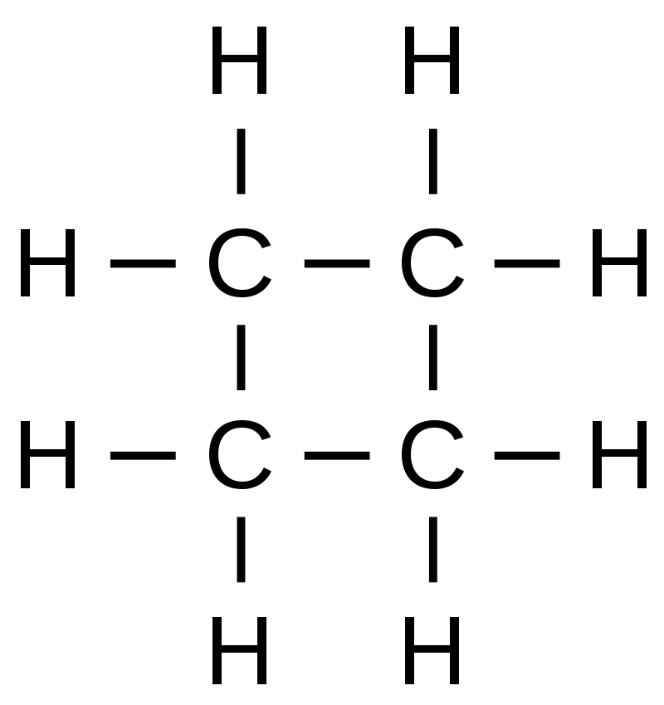
So, from the structure, we can see that it has 8 hydrogen atoms and hence structural formula of cyclobutane will be ${{(C{{H}_{2}})}_{4}}$ or ${{C}_{4}}{{H}_{8}}$.
Additional Information:
Some properties of cyclobutane are
- It is prepared by dimerization of alkenes in UV-light. It can also be prepared by dehalogenation of 1,4-dihalobutene in the presence of reducing metals.
- It is a colorless gas but can be liquified for commercial purposes.
- It has a molar mass of 56.107 g/mol.
- Its boiling point is 285.6K and its melting point is 182K.
- Complex derivatives of cyclobutane are of much importance in the fields of biotechnology and biology.
- It does not have much biological or commercial significance.
- Carbon-carbon bonds in cyclobutane have lower bond energy and have significantly strained bond angles. This is why it is unstable at a temperature above $500{}^\circ C$.
Note:
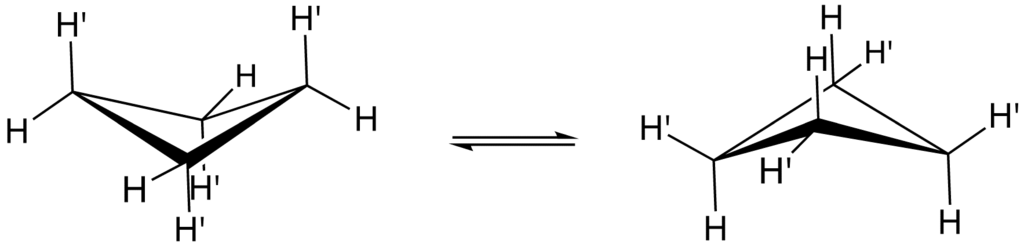
It should be noted that the four carbon atoms in the cyclobutane compound are not coplanar. A puckered or a folded conformation is adopted by the cyclobutane ring. The angle between the plane formed by the three carbon atoms and the fourth carbon atom is $25{}^\circ $.
- It has a cyclic structure
- It has four carbon atoms
- It does not have any multiple bonds
Complete answer:
We know that cyclobutane is a type of cycloalkane. A cycloalkane is a monocyclic saturated hydrocarbon. It does not contain any atoms or elements except hydrogen and carbon.
Since we know that it has a saturated monocyclic structure and has four carbon atoms, its Lewis structure will be as follows

So, from the structure, we can see that it has 8 hydrogen atoms and hence structural formula of cyclobutane will be ${{(C{{H}_{2}})}_{4}}$ or ${{C}_{4}}{{H}_{8}}$.
Additional Information:
Some properties of cyclobutane are
- It is prepared by dimerization of alkenes in UV-light. It can also be prepared by dehalogenation of 1,4-dihalobutene in the presence of reducing metals.
- It is a colorless gas but can be liquified for commercial purposes.
- It has a molar mass of 56.107 g/mol.
- Its boiling point is 285.6K and its melting point is 182K.
- Complex derivatives of cyclobutane are of much importance in the fields of biotechnology and biology.
- It does not have much biological or commercial significance.
- Carbon-carbon bonds in cyclobutane have lower bond energy and have significantly strained bond angles. This is why it is unstable at a temperature above $500{}^\circ C$.
Note:
It should be noted that the four carbon atoms in the cyclobutane compound are not coplanar. A puckered or a folded conformation is adopted by the cyclobutane ring. The angle between the plane formed by the three carbon atoms and the fourth carbon atom is $25{}^\circ $.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




