
How many structural formulas are possible when one of the hydrogen is replaced by a chlorine atom in anthracene?
(A) $ 3 $
(B) $ 7 $
(C) $ 4 $
(D) $ 6 $
Answer
513.9k+ views
Hint :Structural isomers have the same molecular formulas but they have different structures. All the structural isomers have the same number of atoms. But the structural arrangement is different. To solve this question, number all the carbon atoms. This will help us in finding equivalent positions.
Complete Step By Step Answer:
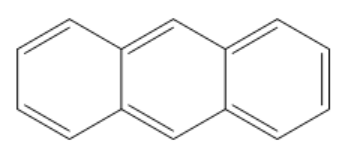
The structure of anthracene is given below:
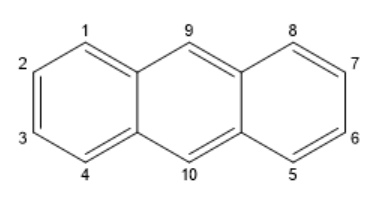
Here, we gave numbers so that no isomers will be repeated.
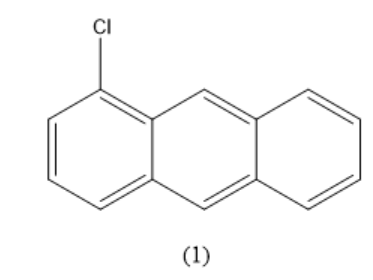
Check the possible places from where we can remove hydrogen atoms. Here, the positions $ 1,4,5,8 $ are equal. It means that if hydrogen is replaced in any of these positions, the resulting compounds will have the same IUPAC name. That means they are similar compounds. So, consider all the four positions equivalent. When one hydrogen atom is replaced by a chlorine atom in this position, the compound formed is given below.
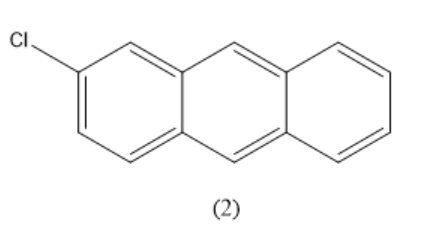
Similarly, the positions $ 2,3,6,7 $ are equal. Consider all these four positions as equivalent. When one hydrogen atom is replaced by a chlorine atom in this position, the compound formed is given below.
Positions $ 9,10 $ are equal. Consider these two positions as equivalent. When one hydrogen atom is replaced by a chlorine atom in this position, the compound formed is given below.
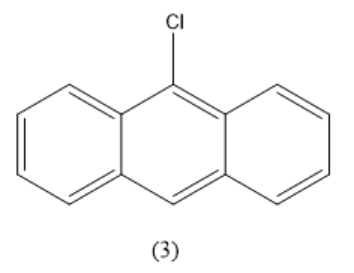
There are three different positions of hydrogen. Hence, three structural isomers are possible.
Therefore, option A is the correct answer.
Note :
Remember that we should count all the possible equivalent positions as one position. Because replacing a hydrogen atom by a chlorine atom in all these positions, gives us the same compound. To identify the same compounds, write the IUPAC names and eliminate compounds having the same IUPAC names.
Complete Step By Step Answer:
The structure of anthracene is given below:

Here, we gave numbers so that no isomers will be repeated.

Check the possible places from where we can remove hydrogen atoms. Here, the positions $ 1,4,5,8 $ are equal. It means that if hydrogen is replaced in any of these positions, the resulting compounds will have the same IUPAC name. That means they are similar compounds. So, consider all the four positions equivalent. When one hydrogen atom is replaced by a chlorine atom in this position, the compound formed is given below.

Similarly, the positions $ 2,3,6,7 $ are equal. Consider all these four positions as equivalent. When one hydrogen atom is replaced by a chlorine atom in this position, the compound formed is given below.

Positions $ 9,10 $ are equal. Consider these two positions as equivalent. When one hydrogen atom is replaced by a chlorine atom in this position, the compound formed is given below.

There are three different positions of hydrogen. Hence, three structural isomers are possible.
Therefore, option A is the correct answer.
Note :
Remember that we should count all the possible equivalent positions as one position. Because replacing a hydrogen atom by a chlorine atom in all these positions, gives us the same compound. To identify the same compounds, write the IUPAC names and eliminate compounds having the same IUPAC names.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




