
Structural isomer in ${C_5}{H_{12}}$
Answer
517.2k+ views
Hint: Isomers are molecules with similar molecular formulas — that is, the same number of atoms of an element — but different atomic configurations in space, according to chemistry. Isomerism refers to the presence or potential of isomers.
Complete answer:
The concept of isomerism occurs when two or more molecules have the same chemical formula but different chemical structures. Isomers are chemical compounds with similar chemical formulae that vary in properties and atom structure in the molecule. As a result, compounds that show isomerism are referred to as isomers.
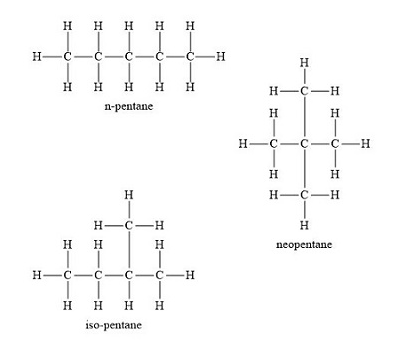
Pentane is an organic compound with the formula \[{C_5}{H_{12}}\] , which means it's a five-carbon alkane. The term pentane may refer to any of three structural isomers, or a mixture of them; however, in IUPAC nomenclature, pentane only refers to the n-pentane isomer; the other two are referred to as isopentane and neopentane, respectively.
1. \[n - Pentane\] is a kind of n-butane (Maximizing the number of carbon atoms in the main chain)
2. \[2 - methylbutane\] or isopentane (Shortening the main chain to four C-atoms and looking for positions to place one methyl group)
3. \[2,2 - dimethylpropane\] or neopentane (Main chain has three carbons in this case).
Pentane can thus be divided into three structural isomers.
Note:
Pentane has a variety of industrial applications in addition to being a part of natural gas. Pentane is mostly used to make a blowing agent, which is then used to make polystyrene foam. Insulation systems for refrigerators and heating pipes are made of polystyrene.
Complete answer:
The concept of isomerism occurs when two or more molecules have the same chemical formula but different chemical structures. Isomers are chemical compounds with similar chemical formulae that vary in properties and atom structure in the molecule. As a result, compounds that show isomerism are referred to as isomers.
Pentane is an organic compound with the formula \[{C_5}{H_{12}}\] , which means it's a five-carbon alkane. The term pentane may refer to any of three structural isomers, or a mixture of them; however, in IUPAC nomenclature, pentane only refers to the n-pentane isomer; the other two are referred to as isopentane and neopentane, respectively.
1. \[n - Pentane\] is a kind of n-butane (Maximizing the number of carbon atoms in the main chain)
2. \[2 - methylbutane\] or isopentane (Shortening the main chain to four C-atoms and looking for positions to place one methyl group)
3. \[2,2 - dimethylpropane\] or neopentane (Main chain has three carbons in this case).
Pentane can thus be divided into three structural isomers.

Note:
Pentane has a variety of industrial applications in addition to being a part of natural gas. Pentane is mostly used to make a blowing agent, which is then used to make polystyrene foam. Insulation systems for refrigerators and heating pipes are made of polystyrene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




