
How many structural isomers of \[{C_5}{H_{10}}O\] are there:
A.4
B.5
C.7
D.6
Answer
583.2k+ views
Hint: Compounds that have the same molecular formula but differ in the arrangement of constituent atoms is called isomers. Two main types of isomerism are structural isomerism and stereoisomerism.
Complete step by step answer:
Isomers will have the similar formula but they will differ in the arrangement of atoms.
For an open chain hydrocarbon there should be at least 4 carbon atoms should be present. C H O is an organic molecule having 5 carbon atoms, 10 H atoms and one O atom. So, it will have isomers.
But there is no direct formula to find the number of isomers of an open chain hydrocarbon. Only way is to draw all the possible structures.
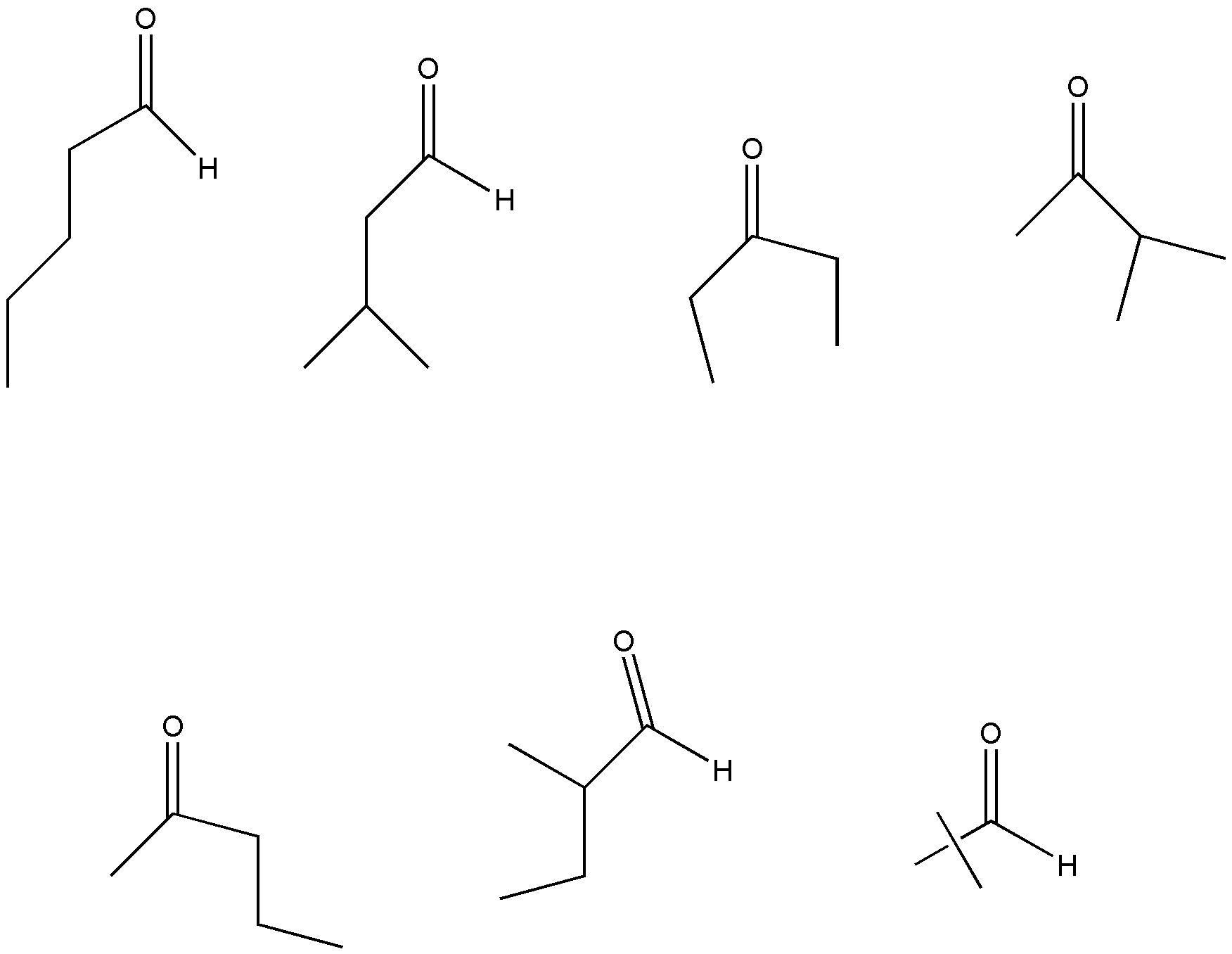
\[{C_5}{H_{10}}O\] have 7 isomers. So, the correct answer is C.
Additional information: Isomers having the same structure and different spatial arrangement of atoms or groups are called stereoisomers. We can calculate the number of stereoisomers using the formula 2n, where n is the number of chiral carbon atoms.
Note: Structural isomerism is again classified as chain isomerism, position isomerism, functional isomerism, metamerism, ring chain isomerism and tautomerism. Isomers differ in the arrangement of the carbon chain within their molecules are called structural isomers. Differ in the position of structural entities such as multiple bonds or functional groups. Functional isomers will differ in the functional group. Isomers differ in the number of alkyl groups attached to the two sides of a bivalent functional group.
Complete step by step answer:
Isomers will have the similar formula but they will differ in the arrangement of atoms.
For an open chain hydrocarbon there should be at least 4 carbon atoms should be present. C H O is an organic molecule having 5 carbon atoms, 10 H atoms and one O atom. So, it will have isomers.
But there is no direct formula to find the number of isomers of an open chain hydrocarbon. Only way is to draw all the possible structures.

\[{C_5}{H_{10}}O\] have 7 isomers. So, the correct answer is C.
Additional information: Isomers having the same structure and different spatial arrangement of atoms or groups are called stereoisomers. We can calculate the number of stereoisomers using the formula 2n, where n is the number of chiral carbon atoms.
Note: Structural isomerism is again classified as chain isomerism, position isomerism, functional isomerism, metamerism, ring chain isomerism and tautomerism. Isomers differ in the arrangement of the carbon chain within their molecules are called structural isomers. Differ in the position of structural entities such as multiple bonds or functional groups. Functional isomers will differ in the functional group. Isomers differ in the number of alkyl groups attached to the two sides of a bivalent functional group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




