
Structure of methyl propyl ether (methoxypropane) is:
A. $CH_3 - CH_2 - CH_3$
B.
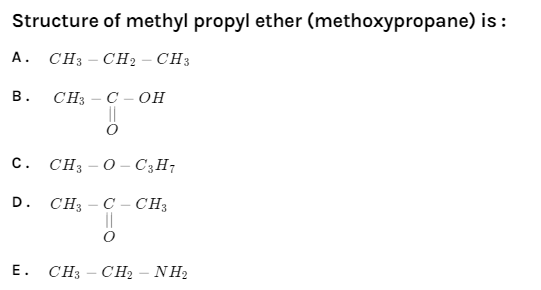
C. $CH_3 - O - C_3H_7$
D.
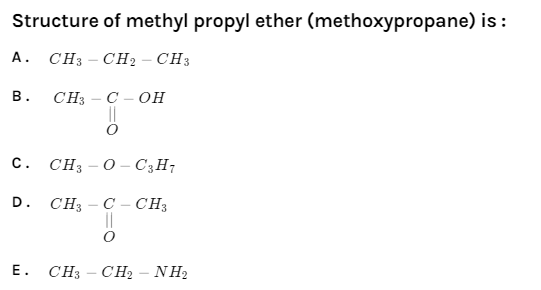
E. $CH_3 - CH_2 - NH_2$
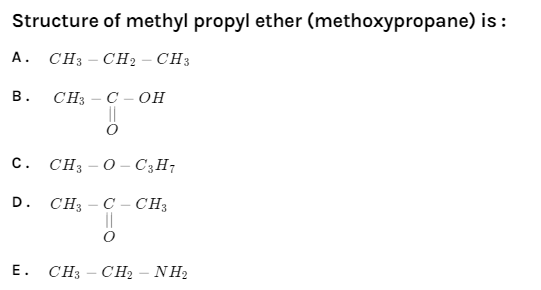
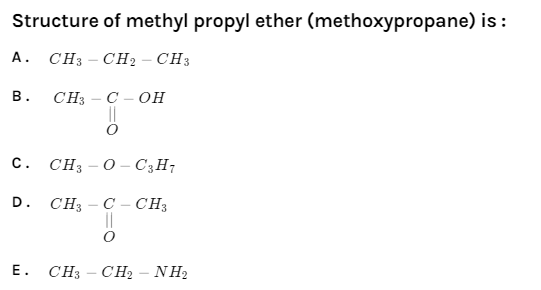
Answer
605.4k+ views
Hint: You can try to identify the functional group present in the name to get an idea about its structure and then count the number of carbon in structure and compare it with the IUPAC name given in question. You will definitely get your answer.
Complete step by step answer:
Here we will check all the options one by one and use the elimination method to find our answer.
Option A, it is a simple alkane because it has no substituent attached to it. Name of this alkane is n-propane.
Option B, it has the functional group carboxylic acid present in it. The name of this acid is ethanoic acid.
Option C contains the functional group ether in its structure. The name of this compound is methoxypropane. Hence, it is our correct answer.
Option D, it has the presence of functional group carbonyl and to be specific it is a ketone. This compound is generally known as Acetone.
Option E, the name of this compound is ethanamine. This compound has the functional group primary amine with two carbons in its structure.
Therefore, the correct answer for this question is option C.
Additional information:
Methoxypropane, or methyl propyl ether, was once used as a general anaesthetic. Marketed under the trade names Metopryl and Neothyl, methoxypropane was used as an alternative to diethyl ether because of its greater potency.
Its use as an anaesthetic has since been supplanted by modern halogenated ethers which are much less flammable.
Note: Here you should not confuse yourself with the nomenclature of ether. It is written as methyl propyl ether, not as propyl methyl ether, because nomenclature of any compound is done on the basis of alphabetical order of alkyl substituent present in the compound.
Complete step by step answer:
Here we will check all the options one by one and use the elimination method to find our answer.
Option A, it is a simple alkane because it has no substituent attached to it. Name of this alkane is n-propane.
Option B, it has the functional group carboxylic acid present in it. The name of this acid is ethanoic acid.
Option C contains the functional group ether in its structure. The name of this compound is methoxypropane. Hence, it is our correct answer.
Option D, it has the presence of functional group carbonyl and to be specific it is a ketone. This compound is generally known as Acetone.
Option E, the name of this compound is ethanamine. This compound has the functional group primary amine with two carbons in its structure.
Therefore, the correct answer for this question is option C.
Additional information:
Methoxypropane, or methyl propyl ether, was once used as a general anaesthetic. Marketed under the trade names Metopryl and Neothyl, methoxypropane was used as an alternative to diethyl ether because of its greater potency.
Its use as an anaesthetic has since been supplanted by modern halogenated ethers which are much less flammable.
Note: Here you should not confuse yourself with the nomenclature of ether. It is written as methyl propyl ether, not as propyl methyl ether, because nomenclature of any compound is done on the basis of alphabetical order of alkyl substituent present in the compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




