
Sucrose is not a reducing sugar because:
A.) It is chemically stable.
B.) It contains no free aldehyde or keto group adjacent to a $\rangle CHOH$ group.
C.) It is built up of a fructose unit.
D.) It is optically active.
Answer
587.7k+ views
Hint: To solve this question we need to know that non reducing sugars are those carbohydrates that does not contain free aldehyde and ketone functional groups in them and sucrose is a non- reducing agent.
Complete step by step answer:
Sucrose is a disaccharide carbohydrate. By disaccharide carbohydrates we mean carbohydrates which is composed of two monosaccharides. The two saccharides from which sucrose is made are glucose and fructose. Sucrose has the molecular formula ${C_{12}}{H_{22}}{O_{11}}$.
Sucrose is formed when $\alpha - D - $Glucose and $\beta - D - $fructose combine and release a water molecule and then the sucrose is obtained. The structure of the sucrose can be given as :
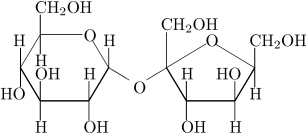
Non- reducing sugars are those sugars which do not have free aldehyde or ketone groups in them. In sucrose, the two monosaccharides that are glucose and fructose are linked together by glycosidic linkage between carbon$ - 1$ of $\alpha - $glucose and carbon$ - 2$ of $\beta - $fructose. As we can see that glucose and fructose are involved in glycosidic bonds and thus sucrose cannot participate in the reaction to get reduced. Hence, sucrose is a non- reducing sugar because of no free aldehyde or ketone adjacent to the $\rangle CHOH$ group.
Hence, option B is the correct answer.
Note:
We should remember that reducing sugars are those sugars which have free aldehyde and ketone groups in them and all monosaccharides are reducing sugars. Also reducing sugars can reduce Fehling’s solution and tollen’s reagent.
Complete step by step answer:
Sucrose is a disaccharide carbohydrate. By disaccharide carbohydrates we mean carbohydrates which is composed of two monosaccharides. The two saccharides from which sucrose is made are glucose and fructose. Sucrose has the molecular formula ${C_{12}}{H_{22}}{O_{11}}$.
Sucrose is formed when $\alpha - D - $Glucose and $\beta - D - $fructose combine and release a water molecule and then the sucrose is obtained. The structure of the sucrose can be given as :

Non- reducing sugars are those sugars which do not have free aldehyde or ketone groups in them. In sucrose, the two monosaccharides that are glucose and fructose are linked together by glycosidic linkage between carbon$ - 1$ of $\alpha - $glucose and carbon$ - 2$ of $\beta - $fructose. As we can see that glucose and fructose are involved in glycosidic bonds and thus sucrose cannot participate in the reaction to get reduced. Hence, sucrose is a non- reducing sugar because of no free aldehyde or ketone adjacent to the $\rangle CHOH$ group.
Hence, option B is the correct answer.
Note:
We should remember that reducing sugars are those sugars which have free aldehyde and ketone groups in them and all monosaccharides are reducing sugars. Also reducing sugars can reduce Fehling’s solution and tollen’s reagent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




