
How will you synthesize polystyrene from benzene?
Answer
514.2k+ views
Hint: Polystyrene is one of the most widely used polymers which is synthesised from styrene. It is a thermoplastic polymer i.e., it becomes moldable when the temperature rises to a certain temperature and solidifies on cooling. Thus, polystyrene can exist in both solid as well as foamed states.
Complete answer: As the monomer of polystyrene is styrene, we need to first convert benzene into styrene then after polymerization reaction, polystyrene will be formed. Therefore, the synthesis of polystyrene from benzene is done as per following steps:
Step-1: Benzene reacts with ethene in the presence of aluminium chloride to give ethyl benzene. The reaction proceeds as follows:
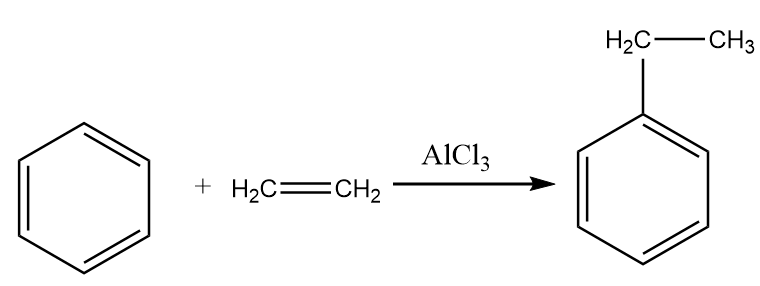
Step-2: Ethyl benzene when heated to ${650^ \circ }C$ in the presence of ferric oxide, then the formation of styrene takes place. The reaction proceeds as follows:
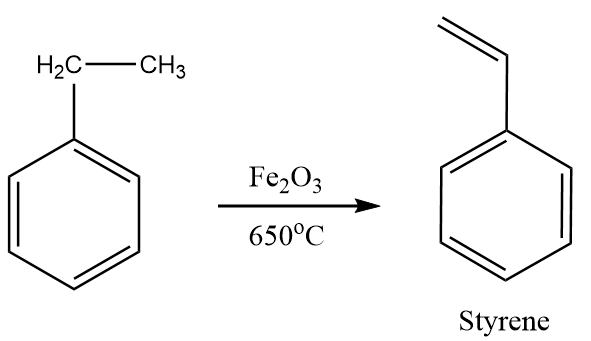
Step-3: Styrene undergoes polymerisation reaction and formation of polystyrene takes place. The reaction proceeds as follows:
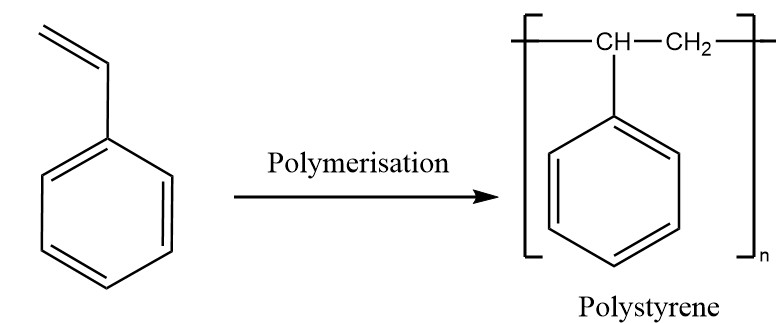
Hence, Polystyrene is synthesised from benzene in three steps.
Note:
It is important to note that the polymerisation reaction of styrene follows a free radical mechanism to form polystyrene. The three steps involved in the process are as follows:
1.Initiation step: In the presence of peroxide, the homolytic cleavage of covalent bond takes place and respective alkyl group free radicals are produced in this step.
2.Propagation step: The free radicals formed react with n molecules of free radical styrene to produce a long chain polymer i.e., Polystyrene.
Complete answer: As the monomer of polystyrene is styrene, we need to first convert benzene into styrene then after polymerization reaction, polystyrene will be formed. Therefore, the synthesis of polystyrene from benzene is done as per following steps:
Step-1: Benzene reacts with ethene in the presence of aluminium chloride to give ethyl benzene. The reaction proceeds as follows:

Step-2: Ethyl benzene when heated to ${650^ \circ }C$ in the presence of ferric oxide, then the formation of styrene takes place. The reaction proceeds as follows:

Step-3: Styrene undergoes polymerisation reaction and formation of polystyrene takes place. The reaction proceeds as follows:

Hence, Polystyrene is synthesised from benzene in three steps.
Note:
It is important to note that the polymerisation reaction of styrene follows a free radical mechanism to form polystyrene. The three steps involved in the process are as follows:
1.Initiation step: In the presence of peroxide, the homolytic cleavage of covalent bond takes place and respective alkyl group free radicals are produced in this step.
2.Propagation step: The free radicals formed react with n molecules of free radical styrene to produce a long chain polymer i.e., Polystyrene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




