
How many tautomers can you draw for the following diketone?
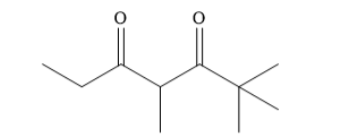
A 1
B 2
C 3
D 4

Answer
569.7k+ views
Hint: A diketone contains two carbonyl groups. If a ketone has an alpha hydrogen atom adjacent to a carbonyl group, then it shows keto-enol tautomerism. If a ketone lacks an alpha hydrogen atom adjacent to a carbonyl group, then it cannot show keto-enol tautomerism.
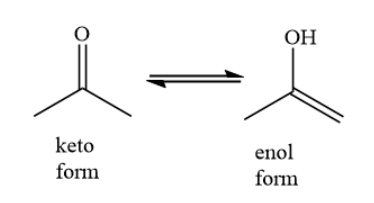
Complete Step by step answer: In keto-enol tautomerism, an equilibrium exists between keto form and enol form. The keto form is a carbonyl group having two alkyl groups. In the enol form, a carbon-carbon double bond and a hydroxyl group is present.
For example, consider the keto enol tautomerism of acetone as shown below:
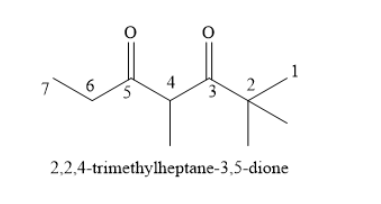
Write the structural formula f the given diketone as shown below:
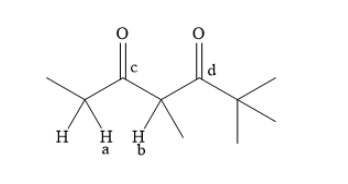
Identify the different alpha hydrogen atoms present in the molecule. Label these alpha hydrogen atoms as ‘a’ and ‘b’. Identify two carbonyl groups present in the molecule. Label these carbonyl groups as ‘c’ and ‘d’.
The tautomerism between the carbonyl group ‘c’ and the alpha hydrogen ‘a’ forms one keto-enol tautomer. The alpha hydrogen ‘a’ now becomes part of the hydroxyl group. A carbon-carbon double bond is formed and a carbon-oxygen double bond is converted into single bond.
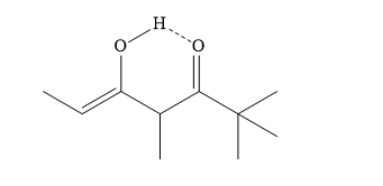
In the above enol form, the hydrogen atom of hydroxyl group of enol forms an intramolecular hydrogen bond with neighboring carbonyl groups. This leads to stabilization of the enol.
The tautomerism between the carbonyl group c and the alpha hydrogen b forms a second keto-enol tautomer. The alpha hydrogen ‘b’ now becomes part of the hydroxyl group. A carbon-carbon double bond is formed and a carbon-oxygen double bond is converted into single bond.
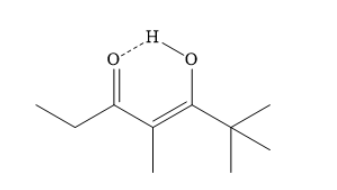
In the above enol form, the hydrogen atom of hydroxyl group of enol forms an intramolecular hydrogen bond with neighboring carbonyl groups. This leads to stabilization of the enol.
Thus, you can draw three tautomers for the given diketone.
Note: A ketone contains carbon oxygen double bond. Two carbon atoms form bonds with carbonyl carbon atoms. An enol contains a carbon – carbon double bond and an oxygen- hydrogen single bond. In enol, the prefix en represents a carbon-carbon double bond and the suffix ol represents a hydroxyl group.
Complete Step by step answer: In keto-enol tautomerism, an equilibrium exists between keto form and enol form. The keto form is a carbonyl group having two alkyl groups. In the enol form, a carbon-carbon double bond and a hydroxyl group is present.
For example, consider the keto enol tautomerism of acetone as shown below:

Write the structural formula f the given diketone as shown below:

Identify the different alpha hydrogen atoms present in the molecule. Label these alpha hydrogen atoms as ‘a’ and ‘b’. Identify two carbonyl groups present in the molecule. Label these carbonyl groups as ‘c’ and ‘d’.

The tautomerism between the carbonyl group ‘c’ and the alpha hydrogen ‘a’ forms one keto-enol tautomer. The alpha hydrogen ‘a’ now becomes part of the hydroxyl group. A carbon-carbon double bond is formed and a carbon-oxygen double bond is converted into single bond.

In the above enol form, the hydrogen atom of hydroxyl group of enol forms an intramolecular hydrogen bond with neighboring carbonyl groups. This leads to stabilization of the enol.
The tautomerism between the carbonyl group c and the alpha hydrogen b forms a second keto-enol tautomer. The alpha hydrogen ‘b’ now becomes part of the hydroxyl group. A carbon-carbon double bond is formed and a carbon-oxygen double bond is converted into single bond.

In the above enol form, the hydrogen atom of hydroxyl group of enol forms an intramolecular hydrogen bond with neighboring carbonyl groups. This leads to stabilization of the enol.
Thus, you can draw three tautomers for the given diketone.
Note: A ketone contains carbon oxygen double bond. Two carbon atoms form bonds with carbonyl carbon atoms. An enol contains a carbon – carbon double bond and an oxygen- hydrogen single bond. In enol, the prefix en represents a carbon-carbon double bond and the suffix ol represents a hydroxyl group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




