
Tell it is Positional Isomers or Chain Isomers.
(1)
(2)
(3)
(4)
(5)






Answer
567.9k+ views
Hint: In positional isomers, the position of the functional group is different in isomeric compounds. Chain isomers have different chain structures in compounds. i.e. carbon skeleton is not the same.
Complete Step-by-Step Solution:
- Positional isomers are the compounds which have the same molecular formula but the functional group is attached at different positions of the carbon chain.
- Chain isomers are compounds which have the same molecular formula but the carbon skeleton of the chain is different.
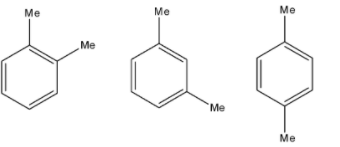
1) - Here, -Me shows methyl groups. We can see that the positions of two methyl groups are different in three compounds. So, they can be called positional isomers.
- The positions of methyl groups in all three compounds are 1,2 , 1,3 , and 1,4 respectively.
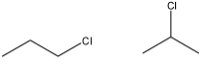
2)- Here in one compound, chlorine group is attached at first carbon while in another compound, the chlorine group is attached at the second carbon of the chain. So, the position of the functional group has changed. Thus, they are positional isomers.
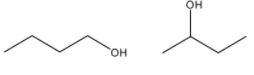
3)- Here, the position of the hydroxyl group is different in both the compounds. In one compound, the hydroxyl group is attached at first carbon while in the other compound, the hydroxyl group is attached with the second carbon. So, they are positional isomers.
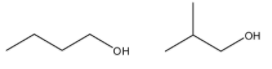
4)- Here, we can see that the chain of carbon atoms is arranged in a different way in both the compounds. However, the molecular formula is the same for both compounds. So, we can call them chain isomers.
5)- Here, in one of the compounds, the cyano group is attached with a straight propyl chain while in the other compound, the cyano group is attached with an isopropyl group.
Thus, we can conclude that pairs given in (4) are chain isomers while (1), (2), (3) and (5) are positional isomers.
Note: Note that chain isomers have different chain structure but we cannot call them positional isomers. Remember that positional isomers always have the same carbon skeleton structure.
Complete Step-by-Step Solution:
- Positional isomers are the compounds which have the same molecular formula but the functional group is attached at different positions of the carbon chain.
- Chain isomers are compounds which have the same molecular formula but the carbon skeleton of the chain is different.
1) - Here, -Me shows methyl groups. We can see that the positions of two methyl groups are different in three compounds. So, they can be called positional isomers.
- The positions of methyl groups in all three compounds are 1,2 , 1,3 , and 1,4 respectively.
2)- Here in one compound, chlorine group is attached at first carbon while in another compound, the chlorine group is attached at the second carbon of the chain. So, the position of the functional group has changed. Thus, they are positional isomers.
3)- Here, the position of the hydroxyl group is different in both the compounds. In one compound, the hydroxyl group is attached at first carbon while in the other compound, the hydroxyl group is attached with the second carbon. So, they are positional isomers.
4)- Here, we can see that the chain of carbon atoms is arranged in a different way in both the compounds. However, the molecular formula is the same for both compounds. So, we can call them chain isomers.
5)- Here, in one of the compounds, the cyano group is attached with a straight propyl chain while in the other compound, the cyano group is attached with an isopropyl group.
Thus, we can conclude that pairs given in (4) are chain isomers while (1), (2), (3) and (5) are positional isomers.
Note: Note that chain isomers have different chain structure but we cannot call them positional isomers. Remember that positional isomers always have the same carbon skeleton structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




