
Terylene is -------
A.An addition polymer with a benzene ring in every repeating unit.
B.A condensation polymer with a benzene ring in every repeating unit.
C.An addition polymer with two carbon atoms in every repeating unit.
D.A condensation polymer with two nitrogen atoms in every repeating unit.
Answer
552k+ views
Hint:Terylene is formed when a molecule of ethylene glycol reacts with Terephthalic acid, these react in such a way that a water molecule gets eliminated thus it is not an additional reaction. Try to figure out the number of atoms by writing the reaction and see the formula made after reaction which is of Terylene.
Complete step-by-step answer:Terylene is condensation polymer which forms when ethylene glycol reacts with Terephthalic acid they give up one water molecule and forms a bond between oxygen and carbon. Terylene is also known as Dacron, thus when sometimes questions arise on Dacron they are asking for Terylene.
Now if we see the reaction, we have to made an ethylene glycol and terephthalic acid, let’s draw them

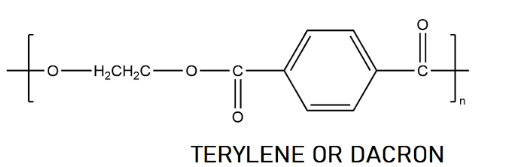
As you see that the produced forms a bond between carbon and oxygen. As there is no nitrogen present so, option D cannot be the right product. If we talk about the option A and C we are seeing from above reaction that a condensation reaction is taking place. These two cannot be the answer.
Option B is correct.
Note:For polymerization reaction we are making a polymer when many molecules of one reactant reacts with (n) number of second reactant. The polymerization happens in the presence of heat. In the above reaction between, we are taking n molecules of ethylene glycol and similarly n molecules of terephthalic acid.
Complete step-by-step answer:Terylene is condensation polymer which forms when ethylene glycol reacts with Terephthalic acid they give up one water molecule and forms a bond between oxygen and carbon. Terylene is also known as Dacron, thus when sometimes questions arise on Dacron they are asking for Terylene.
Now if we see the reaction, we have to made an ethylene glycol and terephthalic acid, let’s draw them


As you see that the produced forms a bond between carbon and oxygen. As there is no nitrogen present so, option D cannot be the right product. If we talk about the option A and C we are seeing from above reaction that a condensation reaction is taking place. These two cannot be the answer.
Option B is correct.
Note:For polymerization reaction we are making a polymer when many molecules of one reactant reacts with (n) number of second reactant. The polymerization happens in the presence of heat. In the above reaction between, we are taking n molecules of ethylene glycol and similarly n molecules of terephthalic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




