
How can you test the presence of proteins in a given food item?
Answer
588.6k+ views
Hint: Almost all reactions involved in testing the presence of proteins in a given food item are chromogenic assays (i.e. colored products are formed).
Complete answer:
The presence of proteins in given foodstuff can be tested as follows:
Method 1: To test protein in the food we need a solution of copper sulfate and caustic soda.
- First we grinded the food sample finely (for example almonds).
- Then, we put it in a clean test tube followed by the addition of 10 drops of water.
- Added 2 drops of copper sulfate solution and shook it well.
- Added 10 drops of caustic soda solution and shook it well.
- The Violet color indicates the presence of proteins.
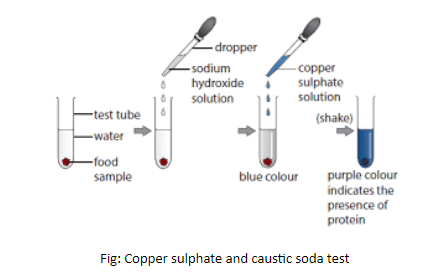
Fig: Copper sulphate and caustic soda test
Method 2: Biuret test –
- First we added the given sample food.
- We then added aqueous copper sulfate.
- The violet coloration confirms the presence of protein.
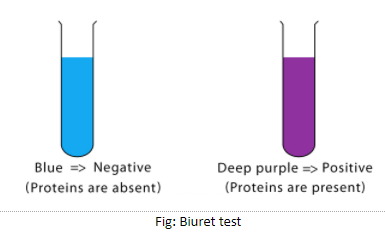
Fig: Biuret test
Method 3: Xanthoproteic test –
- First we added the given sample food.
- We then added the nitric acid.
- The yellow color solution confirms the presence of proteins.
Method 4: Millions test –
- First we added the given sample food.
- We then added the mercuric sulfate in the presence of sodium nitrite and sulfuric acid.
- The brick red color solution confirms the presence of proteins.
Method 5: Ninhydrin test –
- First we added the given sample food.
- We then added the pyridine solution of ninhydrin
- A Violet color solution confirms the presence of proteins.
Note: Biuret reagent solution can be prepared from copper sulfate, sodium/potassium hydroxide, and sodium tartrate. The above mentioned tests can be used for the different food materials and ease of the process.
Complete answer:
The presence of proteins in given foodstuff can be tested as follows:
Method 1: To test protein in the food we need a solution of copper sulfate and caustic soda.
- First we grinded the food sample finely (for example almonds).
- Then, we put it in a clean test tube followed by the addition of 10 drops of water.
- Added 2 drops of copper sulfate solution and shook it well.
- Added 10 drops of caustic soda solution and shook it well.
- The Violet color indicates the presence of proteins.

Fig: Copper sulphate and caustic soda test
Method 2: Biuret test –
- First we added the given sample food.
- We then added aqueous copper sulfate.
- The violet coloration confirms the presence of protein.

Fig: Biuret test
Method 3: Xanthoproteic test –
- First we added the given sample food.
- We then added the nitric acid.
- The yellow color solution confirms the presence of proteins.
Method 4: Millions test –
- First we added the given sample food.
- We then added the mercuric sulfate in the presence of sodium nitrite and sulfuric acid.
- The brick red color solution confirms the presence of proteins.
Method 5: Ninhydrin test –
- First we added the given sample food.
- We then added the pyridine solution of ninhydrin
- A Violet color solution confirms the presence of proteins.
Note: Biuret reagent solution can be prepared from copper sulfate, sodium/potassium hydroxide, and sodium tartrate. The above mentioned tests can be used for the different food materials and ease of the process.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




