
The ABC ABC type packing is called cubic close packing(ccp).If true enter 1 ,else enter 0
Answer
559.2k+ views
Hint: Geometry of a crystal is a regular arrangement of spheres in an infinite. One unit of arrangement is called a lattice. A lattice is an ordered array of points which shows the arrangement of particles that form a crystal. The gap between the two spheres that is the unoccupied space between two spheres is called void.
Complete step by step answer:
In the diagram given below, the blue atoms have been placed above the white (unoccupied) void spaces in layer A. Here, this third layer is displaced horizontally from layer A, let we named it layer C. As we add more layers of atoms, the sequence of layers will become A-B-C-A-B-C-A-B-C...So, it is said to be ABC packing or cubic close packing.
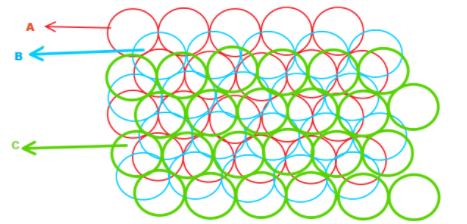
In this arrangement, a substance AB crystallizes in cubic closest packing ( CCP) with B occupying half the tetrahedral voids. And some of which replace the B atoms named as the layer C and remaining occupy few interstitial voids without affecting the dimensions of the cubic crystal lattice.
Note:
There is also another type of cubic structure that FCC is for face centered cubic structures. When the atoms in the octahedral voids the packing is of ABCABC type hence its known as CCP while FCC stands for the unit cell.
Difference between FCC and CCP:
FCC (Face centred cubic) and CCP (Cubic Close Packing) are generally used interchangeably in defining the structure of a compound. FCC is a form of lattice and it does not have determinable properties such as density, volume etc. CCP is a crystal structure form made up of edges and planes and its properties can be determined.
Complete step by step answer:
In the diagram given below, the blue atoms have been placed above the white (unoccupied) void spaces in layer A. Here, this third layer is displaced horizontally from layer A, let we named it layer C. As we add more layers of atoms, the sequence of layers will become A-B-C-A-B-C-A-B-C...So, it is said to be ABC packing or cubic close packing.

In this arrangement, a substance AB crystallizes in cubic closest packing ( CCP) with B occupying half the tetrahedral voids. And some of which replace the B atoms named as the layer C and remaining occupy few interstitial voids without affecting the dimensions of the cubic crystal lattice.
Note:
There is also another type of cubic structure that FCC is for face centered cubic structures. When the atoms in the octahedral voids the packing is of ABCABC type hence its known as CCP while FCC stands for the unit cell.
Difference between FCC and CCP:
FCC (Face centred cubic) and CCP (Cubic Close Packing) are generally used interchangeably in defining the structure of a compound. FCC is a form of lattice and it does not have determinable properties such as density, volume etc. CCP is a crystal structure form made up of edges and planes and its properties can be determined.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




