
The acid used in the making of vinegar is:
A.Formic acid
B.Acetic acid
C.Sulphuric acid
D.Nitric acid
Answer
594k+ views
Hint:
Vinegar is obtained by a process known as fermentation. For producing vinegar, ethyl alcohol or ethanol is fermented by bacteria from the family known as Acetobacteraceae.
Complete step by step answer:
Vinegar can be very well defined as a dilute solution of ethanoic acid in water. Ethanoic acid is also more commonly known as acetic acid.
Vinegar is a naturally occurring compound which is in the liquid state, so there is no defined concentration for it. Although we have a range of about 5% to 20% concentration of acetic acid in water. The chemical formula for vinegar thus obtained is \[C{H_3}COOH\]. So vinegar is basically a diluted solution of acetic acid in water. Because of this, vinegar is considered to be a weak acid. The molecular structure of vinegar can be given as:
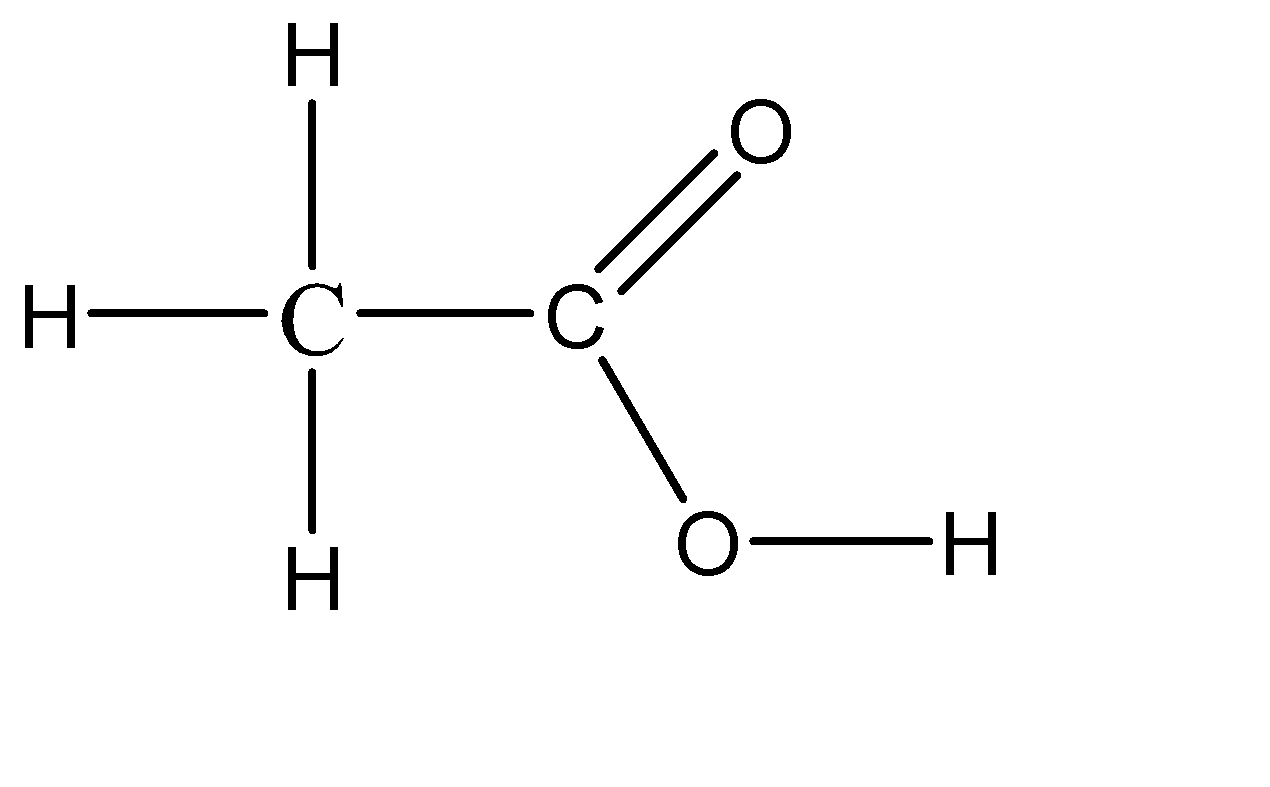
Hence, the acid used in the making of vinegar is acetic acid.
Hence, Option B is the correct option.
Note:
Although the acidity of this compound is low, i.e. it has a very low pH value, even then the acetic acid does not show complete dissociation in water.
Vinegar is obtained by a process known as fermentation. For producing vinegar, ethyl alcohol or ethanol is fermented by bacteria from the family known as Acetobacteraceae.
Complete step by step answer:
Vinegar can be very well defined as a dilute solution of ethanoic acid in water. Ethanoic acid is also more commonly known as acetic acid.
Vinegar is a naturally occurring compound which is in the liquid state, so there is no defined concentration for it. Although we have a range of about 5% to 20% concentration of acetic acid in water. The chemical formula for vinegar thus obtained is \[C{H_3}COOH\]. So vinegar is basically a diluted solution of acetic acid in water. Because of this, vinegar is considered to be a weak acid. The molecular structure of vinegar can be given as:

Hence, the acid used in the making of vinegar is acetic acid.
Hence, Option B is the correct option.
Note:
Although the acidity of this compound is low, i.e. it has a very low pH value, even then the acetic acid does not show complete dissociation in water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




