
The acidity of formic acid is more than acetic acid. Explain.
Answer
565.8k+ views
Hint: Acid is a substance which has the ability to lose hydrogen to form a base. Formic acid is a compound in which a carboxyl group is attached to a hydrogen atom. While acetic acid is a compound in which the carboxyl group is attached to the methyl group.
Complete step by step answer:
The structures of formic acid and acetic acid are given below:
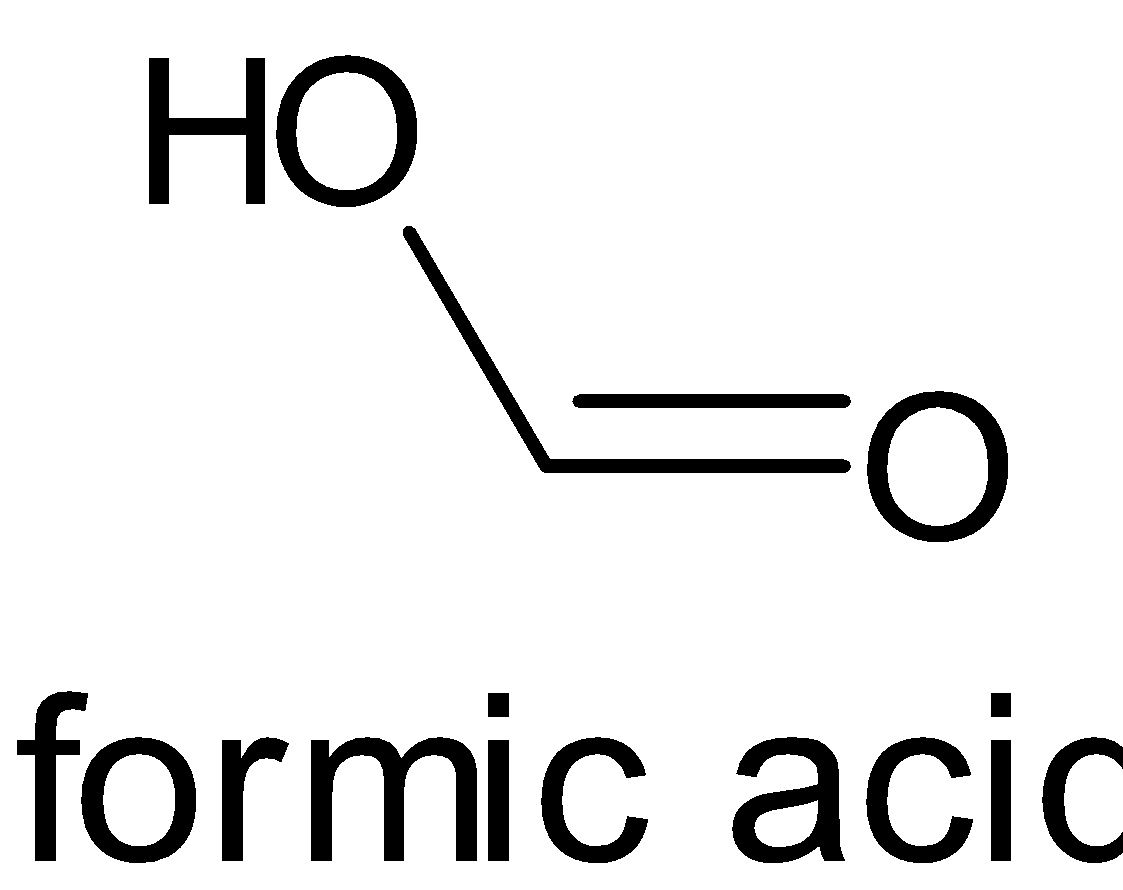
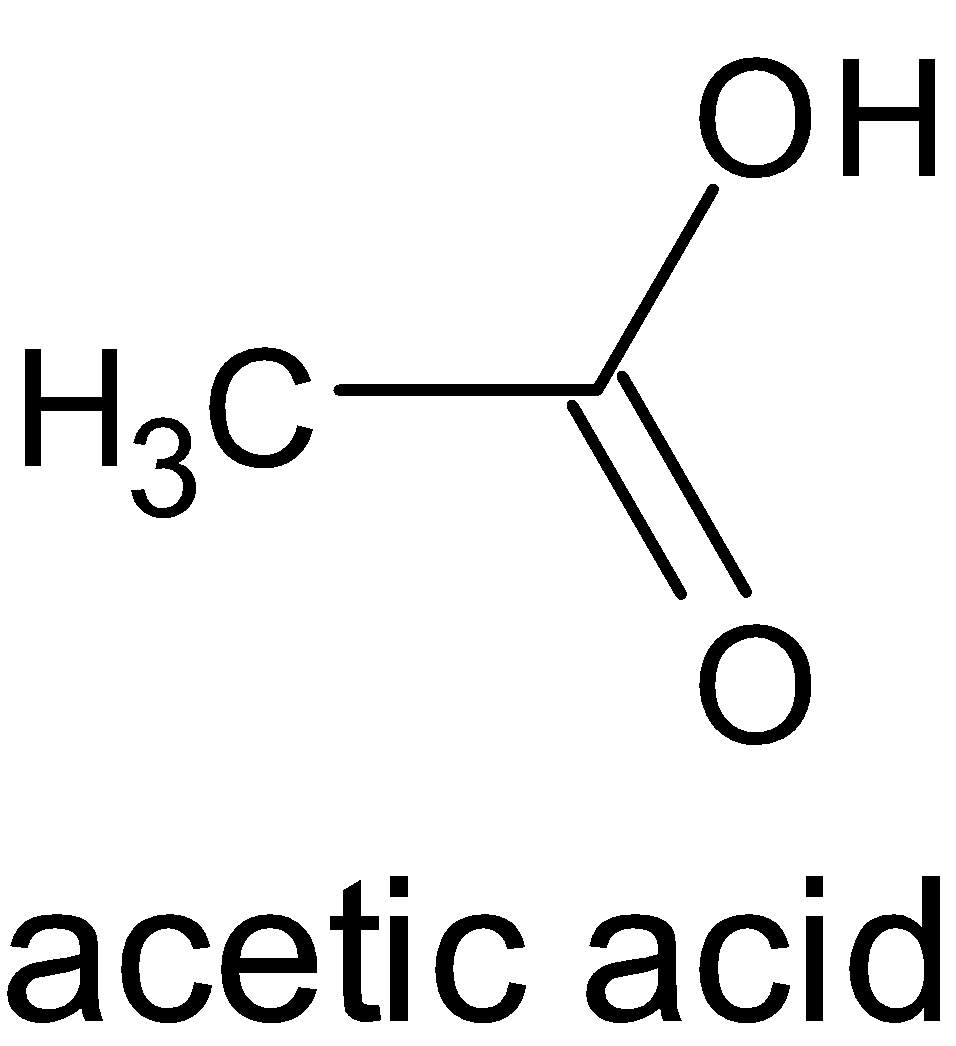
When both compounds are compared, the methyl group is more electron donating than the hydrogen atom. When a methyl group donates electrons, it moves to the carbonyl group and thus creates a negative charge on oxygen atom and a positive charge on carbon atom. But a hydrogen atom does not create any ionization in the compound. This is due to inductive effect, particularly positive inductive effect.
Now let’s consider another factor, i.e. formation of conjugate base. The conjugate base of formic acid, i.e formate anion can be stabilized by induction, resonance and electronegativity. But in acetic acid, its conjugate base, i.e. acetate anion has a methyl group. It has the ability to destabilize the negative charge on the oxygen.
Hence we can say that formic acid is more acidic than acetic acid.
Note: When we consider the theoretical values of acidity of formic acid and acetic acid, ${{pH}}$ is $2.3$ for formic acid and $2.8$ for acetic acid. If the ${{pH}}$ value is more, then the acidity is less. It can also be determined by ${{pKa}}$ value. ${{pKa}}$ of acetic acid is $4.8$ while that of formic acid is $3.8$. If the ${{pKa}}$ value is more, then weaker the acid.
Complete step by step answer:
The structures of formic acid and acetic acid are given below:


When both compounds are compared, the methyl group is more electron donating than the hydrogen atom. When a methyl group donates electrons, it moves to the carbonyl group and thus creates a negative charge on oxygen atom and a positive charge on carbon atom. But a hydrogen atom does not create any ionization in the compound. This is due to inductive effect, particularly positive inductive effect.
Now let’s consider another factor, i.e. formation of conjugate base. The conjugate base of formic acid, i.e formate anion can be stabilized by induction, resonance and electronegativity. But in acetic acid, its conjugate base, i.e. acetate anion has a methyl group. It has the ability to destabilize the negative charge on the oxygen.
Hence we can say that formic acid is more acidic than acetic acid.
Note: When we consider the theoretical values of acidity of formic acid and acetic acid, ${{pH}}$ is $2.3$ for formic acid and $2.8$ for acetic acid. If the ${{pH}}$ value is more, then the acidity is less. It can also be determined by ${{pKa}}$ value. ${{pKa}}$ of acetic acid is $4.8$ while that of formic acid is $3.8$. If the ${{pKa}}$ value is more, then weaker the acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




