
The acidity of the phenol is due to the _ _ _ _ _ _ of its anion. (This question has multiple correct options.)
(a)- Keto-enol tautomerism
(b)- Electron-withdrawing nature
(c)- Hyperconjugation
(d)- Resonance stabilization
Answer
542.4k+ views
Hint: Phenol is an organic compound in which a hydroxyl group is present on the benzene ring, when the hydrogen atom of the phenol is removed then there forms a negative charge on the oxygen atom which will start delocalize from its position to the benzene ring.
Complete answer:
Phenol is an organic compound and it is an aromatic compound. It is a compound in which a hydroxyl group, i.e., -OH is present on the benzene ring. So, specifically, we can say phenol is an aromatic alcohol. The formula is ${{C}_{6}}{{H}_{5}}OH$. The structure is given below:
Phenol is acidic in nature but it is a weak acid. The acidic nature can be discussed as, when the hydrogen atom from the hydroxyl group is removed then there is the formation of phenoxide ion, in which the oxygen atom has a negative charge. Now, this negative charge has the ability to delocalize from its position to the benzene ring. So, resonance is possible in the phenoxide ion, due to which it is more stable than the phenol. The resonating structures are given below:
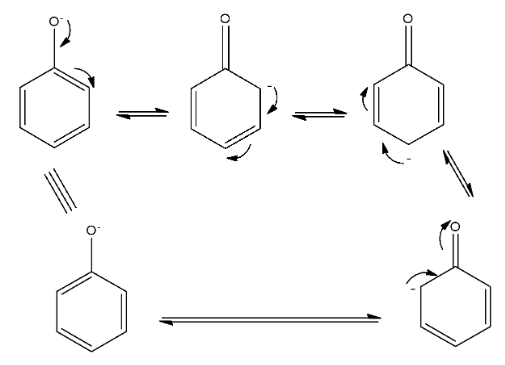
So, due to the resonance stabilization, the phenol is acidic. Also, when the negative charge of the oxygen atom moves towards the ring, there is the formation of the double bond between the oxygen and carbon atom, which is a keto compound. This is due to keto-enol tautomerism.
Therefore, the correct answers are an option (a) and (d).
Note: The double is stronger than the single bond so, the negative charge will easily move from the oxygen atom to the benzene ring. Due to this resonance factor, the aromatic alcohols are more acidic than aliphatic alcohols.
Complete answer:
Phenol is an organic compound and it is an aromatic compound. It is a compound in which a hydroxyl group, i.e., -OH is present on the benzene ring. So, specifically, we can say phenol is an aromatic alcohol. The formula is ${{C}_{6}}{{H}_{5}}OH$. The structure is given below:

Phenol is acidic in nature but it is a weak acid. The acidic nature can be discussed as, when the hydrogen atom from the hydroxyl group is removed then there is the formation of phenoxide ion, in which the oxygen atom has a negative charge. Now, this negative charge has the ability to delocalize from its position to the benzene ring. So, resonance is possible in the phenoxide ion, due to which it is more stable than the phenol. The resonating structures are given below:

So, due to the resonance stabilization, the phenol is acidic. Also, when the negative charge of the oxygen atom moves towards the ring, there is the formation of the double bond between the oxygen and carbon atom, which is a keto compound. This is due to keto-enol tautomerism.
Therefore, the correct answers are an option (a) and (d).
Note: The double is stronger than the single bond so, the negative charge will easily move from the oxygen atom to the benzene ring. Due to this resonance factor, the aromatic alcohols are more acidic than aliphatic alcohols.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




